3-chloro-2-[3-(trifluoromethyl)phenoxy]pyridine synthesis
- Product Name:3-chloro-2-[3-(trifluoromethyl)phenoxy]pyridine
- CAS Number:197565-66-5
- Molecular formula:C12H7ClF3NO
- Molecular Weight:273.64
Yield:197565-66-5 67%
Reaction Conditions:
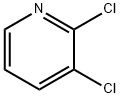
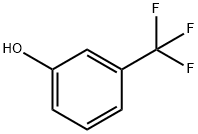
Stage #1: 3-Trifluoromethylphenolwith sodium hydride in N,N-dimethyl-formamide at 20; for 0.5 h;
Stage #2: 2,3-dichloro-pyridine in N,N-dimethyl-formamide at 120; for 5 h;
Steps:
2 3-Chloro-2-[3-(trifluoromethyl)phenoxy]pyridine
EXAMPLE 2 3-Chloro-2-[3-(trifluoromethyl)phenoxy]pyridine 20 7.68 g of sodium hydride dispersion (ca. 50 percent in mineral oil) was washed with pentane under nitrogen and 100 ml of N,N-dimethylformamide was then added. 21.92 g (135 mmol) of 3-(trifluoromethyl)phenol was added dropwise over 30 minutes at room temperature. The resultant phenate solution was added dropwise over 2 hours, under nitrogen, to a solution of 20.1 g (136 mmol) of 2,3-dichloropyridine in 80 ml of N,N-dimethylformamide, heated to 120° C. After a reaction time of 3 hours, the mixture was cooled to room temperature, the precipitate of sodium chloride was filtered off and the filtrate was concentrated. The residue was extracted with toluene and 0.1 N hydrochloric acid, and the organic was washed with saturated sodium chloride solution and concentrated. The oily residue was distilled under vacuum. The yield of the title compound was 24.75 g (67 percent) of a colorless oil, content (GC) 99.7 percent. Other data concerning the title compound was: B.P.18mbar=145°-148° C. nD20=1.5282; MS; m/z: 273/275; 1H NMR (CDCl3): δ=6.99 (m, 1H); 7.36 (d, 1H); 7.45-7.53 (m, 3H); 7.77 (d, 1H); 8.02 (d, 1H). 13C NMR (CDCl3): δ=118.66 (CH); 119.44 (C); 119.98 (CH); 121.75 (CH); 123.78 (CF3); 124.94 (CH); 130.13 (CH); 132.16 (CCF3); 139.65 (CH); 145.20 (CH); 153.88 (C); 158.51 (C).
References:
US6635766,2003,B1 Location in patent:Page/Page column 6