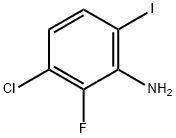
3-chloro-2-fluoro-4-iodoaniline synthesis
- Product Name:3-chloro-2-fluoro-4-iodoaniline
- CAS Number:874840-61-6
- Molecular formula:C6H4ClFIN
- Molecular Weight:271.46
Yield:874840-61-6 63% ,1268684-06-5 10%
Reaction Conditions:
with N-iodo-succinimide in dichloromethane at 20; for 96 h;
Steps:
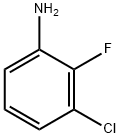
6.a a) A: 3-Chloro-2-fluoro-4-indoaniline, B: 3-Chloro-2-fluoro-6-indoaniline
To a solution of 3-chloro-2-fluoroaniline (13.10 g, 90.0 mmol) in dichloromethane (300 mL), N-iodosuccinimide (21.26 g, 94.5 mmol) was added, and the resulting mixture was stirred at room temperature for 4 days. After the reaction, ethyl acetate (40 mL) and hexane (200 mL) were added to the reaction mixture, and the insoluble matter was separated by filtration. The resulting filtrate was purified by silica gel column chromatography to obtain the title compound A (15.43 g, yield 63%), and the title compound B (2.76 g, yield 10%).A: 3-Chloro-2-fluoro-4-indoaniline1H-NMR (CDCl3) δ: 3.84 (2H, br), 6.48 (1H, t, J=8.2 Hz), 7.36 (1H, dd, J=2.0 Hz, 8.2 Hz)B: 3-Chloro-2-fluoro-6-indoaniline1H-NMR (CDCl3) δ: 4.24 (2H, br), 6.54 (1H, dd, J=6.9 Hz, 8.6 Hz), 7.33 (1H, dd, J=2.0 Hz, 8.6 Hz)
References:
US2017/298081,2017,A1 Location in patent:Paragraph 0195-0200