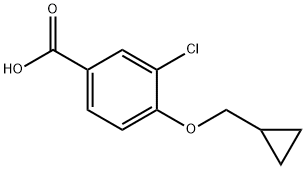
3-chloro-4-(cyclopropylmethoxy)Benzoic acid synthesis
- Product Name:3-chloro-4-(cyclopropylmethoxy)Benzoic acid
- CAS Number:856165-89-4
- Molecular formula:C11H11ClO3
- Molecular Weight:226.66
3964-57-6
175 suppliers
$5.00/250mg

7051-34-5
416 suppliers
$6.00/1g

856165-89-4
27 suppliers
$112.00/250mg
Yield:856165-89-4 11.7 g
Reaction Conditions:
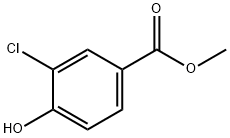
Stage #1: methyl 4-hydroxy-3-chlorobenzoate;cyclopropylcarbinyl bromidewith potassium carbonate in N,N-dimethyl-formamide at 70; for 1 h;
Stage #2: with water;sodium hydroxide in tetrahydrofuran;methanol at 50; for 2 h;
Steps:
29.A A) 3-chloro-4-(cyclopropylmethoxy)benzoic Acid
A)
3-chloro-4-(cyclopropylmethoxy)benzoic Acid
To a solution of methyl 3-chloro-4-hydroxybenzoate (10.0 g) in DMF (100 mL) were added potassium carbonate (14.8 g) and (bromomethyl)cyclopropane (7.80 mL), and the mixture was stirred with heating at 70° C. for 1 hr.
The reaction mixture was allowed to cool to room temperature, diluted with ethyl acetate, and washed with saturated brine.
The obtained organic layer was subjected to silica gel column chromatography (NH, ethyl acetate), and the solvent was evaporated.
To a solution of the obtained residue in a mixed solvent of THF (50 mL) and methanol (50 mL) was added 2 M aqueous sodium hydroxide solution (54 mL), and the mixture was stirred with heating at 50° C. for 2 hr.
The reaction mixture was allowed to cool to room temperature, and neutralized with 6 M hydrochloric acid, and the mixture was extracted with ethyl acetate.
The obtained organic layer was washed with saturated brine, and dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure.
The obtained solid was washed with ethyl acetate to give the title compound (11.7 g).
1H NMR (300 MHz, CDCl3) δ 0.38-0.47 (2H, m), 0.64-0.75 (2H, m), 1.24-1.43 (1H, m), 3.97 (2H, d, J=6.8 Hz), 6.93 (1H, d, J=8.7 Hz), 7.97 (1H, dd, J=8.5, 2.1 Hz), 8.12 (1H, d, J=1.9 Hz).
References:
US2014/243310,2014,A1 Location in patent:Paragraph 1045; 1046