
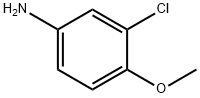
(3-CHLORO-4-METHOXY-PHENYL)-HYDRAZINE synthesis
- Product Name:(3-CHLORO-4-METHOXY-PHENYL)-HYDRAZINE
- CAS Number:24630-85-1
- Molecular formula:C7H9ClN2O
- Molecular Weight:172.61
5345-54-0
114 suppliers
$14.14/1gm:

24630-85-1
21 suppliers
inquiry
Yield:24630-85-1 30.77%
Reaction Conditions:
Stage #1: 3-chloro-4-methoxyanilinewith hydrogenchloride;sodium nitrite in water at 0;
Stage #2: with hydrogenchloride;tin(ll) chloride in water; for 2 h;Cooling;
Stage #3: with water;potassium carbonateCooling;
Steps:
7.12.33. 1-(3-Chloro-4-methoxyphenyl)hydrazine (21)
3-Chloro-4-methoxyaniline (20 g, 0.13 mol) was mixed with concentrated hydrochloric acid (23.4 mL) and treated at 0 °C with a solution of sodium nitrite (8.75 g, 0.13 mol) in (36.9 mL) of water. A well cooled solution of stannous chloride (85.89 g in 74.1 mL of concentrated hydrochloric acid) was added to the reaction solution and allowed to stand for 2 h. After filtration, the reaction mixture was shaken with cold caustic potash solution (164.5 mL, 25%) producing a crystalline solid. The crystalline solid was washed with water and dried in a vacuum desiccator. Recrystallization of the crude mass with (1:3 benzene/hexane) afforded 21 (6.74 g, 30.77%) as yellow crystalline solid. 1H NMR (400 MHz, DMSO-d6): δ 3.71 (s, 3H), 3.94 (s, 2H), 6.53 (s, 1H), 6.68 (dd, 1H, J = 2.4, 8.8 Hz), 6.87 (d, 1H, J = 2.4 Hz), 6.92 (d, 1H, J = 8.8 Hz).
References:
Xie, Jin;Poda, Gennadiy I.;Hu, Yiding;Chen, Natalie X.;Heier, Richard F.;Wolfson, Serge G.;Reding, Matthew T.;Lennon, Patrick J.;Kurumbail, Ravi G.;Selness, Shaun R.;Li, Xiong;Kishore, Nandini N.;Sommers, Cynthia D.;Christine, Lori;Bonar, Sheri L.;Venkatraman, Neetu;Mathialagan, Sumathy;Brustkern, Sarah J.;Huang, Horng-Chih [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 3,p. 1242 - 1255] Location in patent:experimental part