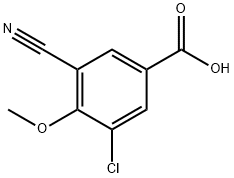
3-Chloro-5-cyano-4-Methoxybenzoic acid synthesis
- Product Name:3-Chloro-5-cyano-4-Methoxybenzoic acid
- CAS Number:1092308-48-9
- Molecular formula:C9H6ClNO3
- Molecular Weight:211.6

65841-11-4
3 suppliers
inquiry

1092308-48-9
4 suppliers
inquiry
Yield:1092308-48-9 946 mg
Reaction Conditions:
with lithium hydroxyde monohydrate in tetrahydrofuran;lithium hydroxide monohydrate at 20; for 1.5 h;
Steps:
(c) 3-Chloro-5-cyano-4-methoxybenzoic acid
Methyl 3-chloro-5-cyano-4-methoxybenzoate (1.02 g) was dissolved in tetrahydrofuran (15 mL) and water (6 mL), lithium hydroxide monohydrate (759 mg) was added. After the mixture was stirred for 90 minutes at room temperature, the organic solvent was evaporated, and the aqueous layer was washed with n-hexane. The aqueous layer was acidified with 1M hydrochloric acid and extracted with ethyl acetate. The organic layer was washed with brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to afford the title compound (946 mg) as colorless crystals. 1H-NMR (DMSO-d6) δ: 4.43 (3H, s), 8.55 (2H, s), 14.00 (1H, brs). MS (m/z): 210 (M-H)-, 212 (M+2-H)-.
References:
Uda, Junichiro;Kobashi, Seiichi;Ashizawa, Naoki;Matsumoto, Koji;Iwanaga, Takashi [Bioorganic and Medicinal Chemistry Letters,2021,vol. 40,art. no. 127900] Location in patent:supporting information