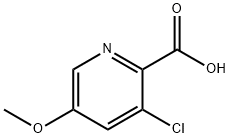
3-Chloro-5-Methoxy-2-pyridinecarboxylic acid synthesis
- Product Name:3-Chloro-5-Methoxy-2-pyridinecarboxylic acid
- CAS Number:128073-09-6
- Molecular formula:C7H6ClNO3
- Molecular Weight:187.58
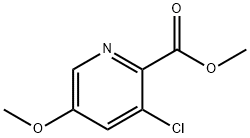
128073-14-3
5 suppliers
inquiry

128073-09-6
28 suppliers
inquiry
Yield:128073-09-6 100%
Reaction Conditions:
with methanol;sodium hydroxide in water at 50; for 1.5 h;
Steps:
39.2 Synthesis of 3-chloro-5-methoxypicolinic acid
In a 2-L round bottom flask, the methyl 3-chloro-5-methoxypicolinate (19.1 g, 95 mmol) was suspended in MeOH (104 ml). Aqueous 1N sodium hydroxide (104 ml, 104 mmol) was added. An air-cooled condenser was attached, and the reaction flask was heated in a 50° C. oil bath. After 1.5 h, the reaction was concentrated to remove the MeOH until a yellow sludge remained. The sludge was dissolved in 110 ml of water. The aqueous phase was extracted with diethyl ether (50 ml), which was discarded. The aqueous phase was acidified with aqueous hydrogen chloride (23 ml, 114 mmol), which caused extensive precipitation. The sludgy aqueous mixture was extracted with DCM in 6×250 ml. The organic layer were dried over MgSO4 and concentrated to yield 3-chloro-5-methoxypicolinic acid (17.88 g, 95 mmol, 100%) as a yellow solid. MS m/z=188 (M+H).
References:
US2014/213581,2014,A1 Location in patent:Paragraph 0765; 0767
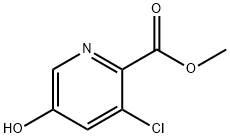
1256811-09-2
27 suppliers
$240.00/250mg

128073-09-6
28 suppliers
inquiry