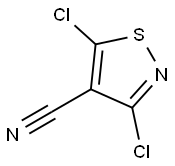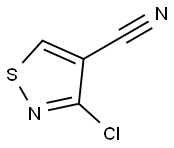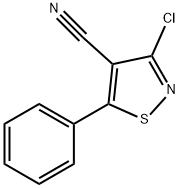
3-CHLORO-5-PHENYLISOTHIAZOLE-4-CARBONITRILE, 97 synthesis
- Product Name:3-CHLORO-5-PHENYLISOTHIAZOLE-4-CARBONITRILE, 97
- CAS Number:28989-23-3
- Molecular formula:C10H5ClN2S
- Molecular Weight:220.68
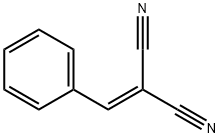
2700-22-3
105 suppliers
$14.00/1g

28989-23-3
15 suppliers
inquiry
Yield: 70.4%
Reaction Conditions:
with pyridine;disulfur dichloride at 140; for 16 h;
Steps:
55
[0739] to a mixture of compound 96a (17.50 g, 113.51 mmol) and chlorosulfanyl thiohypochlorite (70.00 g, 518.36 mmol, 41.42 ml) was added pyridine (900.00 mg, 11.38 mmol). Then the reaction was stirred at 140°c for 16h. The reaction mixture was cooled to room temperature and quenched with ice/H2O (200 ml) and EtOAc (500 ml), yellow solid was was precipitate out, filtered and the filtrate was extracted with EtOAc(100 ml x 2), the combined organic was washed with brine (100 ml), dried over Na2SO4, filtered and the filtrate was concentrated in vacuo. The residue was purified by flash silica gel chromatography (isco; 120 g sepaflash silica flash column, eluent of 0 ~ 10% ethyl acetate/petroleum ether gradient 50 ml/min) to give compound 96b (21.00 g, 70.4% yield) as light yellow solid. 1H NMR (400mhz, chloroform-d) δ 7.77 (br d, 7=7.1 hz, 2h), 7.65 - 7.53 (m, 3h).
References:
BLADE THERAPEUTICS, INC.;BUCKMAN, Brad, Owen;YUAN, Shendong;ADLER, Marc;EMAYAN, Kumaraswamy;MA, Jingyuang WO2018/64119, 2018, A1 Location in patent:Paragraph 0739