
3-CHLORO-5-PHENYLPYRIDINE synthesis
- Product Name:3-CHLORO-5-PHENYLPYRIDINE
- CAS Number:292068-12-3
- Molecular formula:C11H8ClN
- Molecular Weight:189.64
Yield:292068-12-3 68%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);sodium carbonate in ethanol;water;toluene at 80; for 8 h;Inert atmosphere;
Steps:
4 4. Synthesis of Compound 38 (Synthesis of Compound C)
Under an argon (Ar) atmosphere, 5.0 g of 3,5-dichloropyridine, 4.0 g of phenylboronic acid, 3.9 g of tetrakis(triphenylphosphine)palladium(0), and 7.1 g of sodium carbonate were added to a 500 ml, three-neck flask, followed by heating and stirring in 180 ml of a mixed solvent of toluene, water and ethanol (10:2:1) at about 80°C. for about 8 hours. After cooling in air, water was added, an organic layer was separated and taken, the organic layer was dried with magnesium sulfate, and solvents were evaporated under reduced pressure. The crude product thus obtained was separated by silica gel column chromatography to obtain 4.3 g (yield 68%) of Compound C . The molecular weight of Compound C measured by FAB-MS was 189.
References:
US2019/19959,2019,A1 Location in patent:Paragraph 0148; 0149
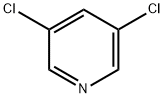
2457-47-8
297 suppliers
$6.00/5g

98-80-6
713 suppliers
$5.00/5g

92-52-4
383 suppliers
$9.00/5g

292068-12-3
18 suppliers
inquiry
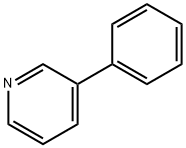
1008-88-4
214 suppliers
$22.00/5g

292068-12-3
18 suppliers
inquiry