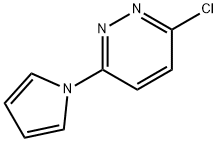
3-CHLORO-6-PYRROL-1-YL-PYRIDAZINE synthesis
- Product Name:3-CHLORO-6-PYRROL-1-YL-PYRIDAZINE
- CAS Number:5096-76-4
- Molecular formula:C8H6ClN3
- Molecular Weight:179.61
Yield:5096-76-4 83%
Reaction Conditions:
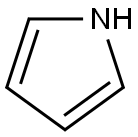
Stage #1: pyrrolewith sodium hydride in N,N-dimethyl-formamide at 20; for 0.0833333 h;
Stage #2: 3,6-dichlorpyridazine in N,N-dimethyl-formamide at 20; for 4 h;
Steps:
General procedure for the preparation of chloro-pyrrolo and chloro-imidazolodiazines 3m, 3n, 5m, 5n, 9m, 9n
General procedure: To a solution pyrrole or imidazole (8.05 mmol) in DMF (10 mL) in a 50 mL round-bottom flask was added sodium hydride (8.86 mmol of NaH 60% dispersion in mineral oil). The mixture was stirred for 5 min at room temperature (H2 evolution). This solution was then transferred dropwise via a syringe to another 50 mL round-bottom flask containing dichlorodiazine (1.00 g, 6.71 mmol) in DMF (5 mL), and the resulting solution was stirred at room temperature (except for 3,6-dichloropyridazine and imidazole at 80 C). The reaction was monitored by GC. Once the starting dichlorodiazine was completely consumed, the mixture was poured into a saturated NH4Cl solution (30 mL) and extracted with CH2Cl2 (330 mL). The combined organic layers were dried over Na2SO4, filtered, and evaporated under vacuum. The crude product was purified by flash chromatography (elution with petroleum ether/acetone 95/5 to 80/20) to afford the pure product.
References:
Sengmany, Stéphane;Lebre, Julie;Le Gall, Ewan;Léonel, Eric [Tetrahedron,2015,vol. 71,# 29,p. 4859 - 4867]