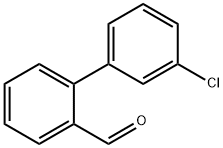
3'-CHLORO-BIPHENYL-2-CARBALDEHYDE synthesis
- Product Name:3'-CHLORO-BIPHENYL-2-CARBALDEHYDE
- CAS Number:216443-25-3
- Molecular formula:C13H9ClO
- Molecular Weight:216.66
108-37-2
355 suppliers
$6.00/10g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

216443-25-3
45 suppliers
$165.00/0.5g
Yield: 43%
Reaction Conditions:
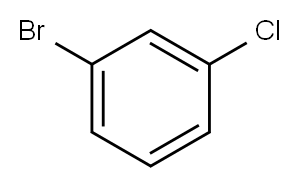
Stage #1:1-bromo-3-chlorobenzene;N-tert-butyl-1-(2-methoxyphenyl)methanimine with chromium dichloride;magnesium in tetrahydrofuran at 20; for 12 h;Inert atmosphere;Schlenk technique;
Stage #2: with hydrogenchloride in tetrahydrofuran at 20; for 0.5 h;Inert atmosphere;Schlenk technique;regioselective reaction;
Steps:
Biphenyl-2-carbaldehyde (3a)
General procedure: In a dried Schlenk tube were placed an ortho-methoxy-bearing aromatic aldimine 1 (0.2 mmol), Mg (11 mg, 0.44 mmol) and CrCl2 (3 mg, 0.02 mmol), then aryl bromide (0.4 mmol)was added by a syringe under atmosphere of nitrogen. After that, 2 ml THF was added and themixture was stirred at room temperature for 12 h. After quenched by 3 N HCl (1 mL), theresulting mixture was further stirred at room temperature for another 0.5 h, and then extractedwith ethyl acetate (3 x 10 mL). The combined organic layer was dried over anhydrous Na2SO4and concentrated under vacuum. The crude product was purified by silica gel chromatographyto afford the desired product 3.
References:
Tang, Jinghua;Luo, Meiming;Zeng, Xiaoming [Synlett,2017,vol. 28,# 19,p. 2577 - 2580] Location in patent:supporting information
63503-60-6
426 suppliers
$9.00/1g
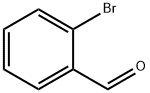
6630-33-7
406 suppliers
$9.00/10g

216443-25-3
45 suppliers
$165.00/0.5g

6630-33-7
406 suppliers
$9.00/10g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

216443-25-3
45 suppliers
$165.00/0.5g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
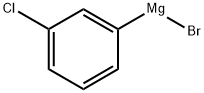
36229-42-2
67 suppliers
$186.00/50ml

216443-25-3
45 suppliers
$165.00/0.5g

579-72-6
73 suppliers
$45.00/100mg

216443-25-3
45 suppliers
$165.00/0.5g