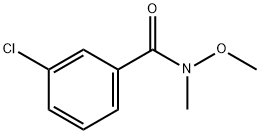
3-CHLORO-N-METHOXY-N-METHYLBENZAMIDE synthesis
- Product Name:3-CHLORO-N-METHOXY-N-METHYLBENZAMIDE
- CAS Number:145959-21-3
- Molecular formula:C9H10ClNO2
- Molecular Weight:199.63
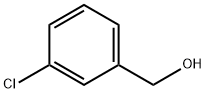
873-63-2
195 suppliers
$6.00/5g

6638-79-5
557 suppliers
$6.00/25g

145959-21-3
69 suppliers
$52.50/250mg
Yield:145959-21-3 90%
Reaction Conditions:
with tert.-butylhydroperoxide;copper(II) acetate monohydrate;calcium carbonate in acetonitrile at 80; for 24 h;
Steps:
Oxidative Synthesis of Weinreb Amides 3 from Benzyl Alcohols 1 and N,O-Dimethylhydroxylamine Hydrochloride Salt (2); General Procedure
General procedure: An oven-dried 15 mL glass vial with a magnetic stirrer bar was charged with Cu(OAc)2·H2O (12 mg, 6 mol%), N,O-dimethylhydroxylamine hydrochloride (2; 117 mg, 1.2 mmol), the respective benzyl alcohol 1 (1 mmol), aq 70% TBHP (0.17 mL, 1.2 mmol), CaCO3 (120 mg, 1.2 mmol) in MeCN (1 mL). The glass vial was flushed with N2 three times and the contents were stirred at r.t. for 1 h. Then the reaction mixture was stirred for 24 h at 80 °C. After completion of the reaction, the mixture was cooled to r.t. All volatiles were removed under vacuum. The product was extracted with EtOAc (20 mL) and the organic layer was washed with sat. aq NaHCO3 (20 mL), dried (Na2SO4), and the solvent removed under vacuum. The Weinreb amide product 3 was purified by column chromatography (silica gel, 100-200 mesh) using a gradient of petroleum ether (bp 60-80 °C) and EtOAc. All the amides were identified by GC-MS, 1H, and 13C NMR spectroscopic analysis.
References:
Yedage, Subhash L.;Bhanage, Bhalchandra M. [Synthesis,2015,vol. 47,# 4,art. no. SS-2014-T0513-OP,p. 526 - 532]

6638-79-5
557 suppliers
$6.00/25g
535-80-8
393 suppliers
$5.00/5g

145959-21-3
69 suppliers
$52.50/250mg

6638-79-5
557 suppliers
$6.00/25g
618-46-2
344 suppliers
$9.00/25g

145959-21-3
69 suppliers
$52.50/250mg

618-46-2
344 suppliers
$9.00/25g

1117-97-1
69 suppliers
inquiry

145959-21-3
69 suppliers
$52.50/250mg
201230-82-2
1 suppliers
inquiry
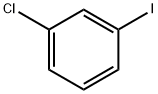
625-99-0
217 suppliers
$5.00/1g

6638-79-5
557 suppliers
$6.00/25g

145959-21-3
69 suppliers
$52.50/250mg