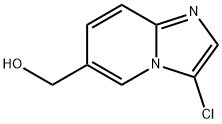
(3-ChloroiMidazo[1,2-a]pyridin-6-yl)Methanol synthesis
- Product Name:(3-ChloroiMidazo[1,2-a]pyridin-6-yl)Methanol
- CAS Number:167884-21-1
- Molecular formula:C8H7ClN2O
- Molecular Weight:182.61
![6-(Hydroxymethyl)imidazo[1,2-a]pyridine](/CAS/GIF/132213-07-1.gif)
132213-07-1
87 suppliers
$33.00/100mg
![(3-ChloroiMidazo[1,2-a]pyridin-6-yl)Methanol](/CAS/20150408/GIF/167884-21-1.gif)
167884-21-1
12 suppliers
inquiry
Yield:167884-21-1 68%
Reaction Conditions:
with N-chloro-succinimide in acetonitrile; for 4 h;
Steps:
18.a
To a suspension of imidazo[l,2-α]pyridin-6-ylmethanol (410 mg, 2.77 mmol, Maybridge) in acetonitrile (14 mL) was added NCS (388 mg, 2.91 mmol) in one portion. After 4 h, the reaction mixture was concentrated under reduced pressure and the residue was triturated with boiling 95:5 v/v EtOAc/MeOH and hot filtered. The filtrate was purified directly by silica gel chromatography (95:5 EtOAc/MeOH) to give 343 mg (68%) of (3-chloroimidazo[l,2-α]pyridin-6-yl)methanol as a pale yellow solid. MS(ES)+ m/e 182.7 [M+H]+.
References:
WO2008/14219,2008,A2 Location in patent:Page/Page column 56