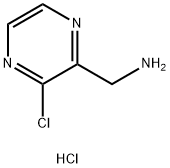
(3-Chloropyrazin-2-yl)MethanaMine hydrochloride synthesis
- Product Name:(3-Chloropyrazin-2-yl)MethanaMine hydrochloride
- CAS Number:939412-86-9
- Molecular formula:C5H7Cl2N3
- Molecular Weight:180.03
55557-52-3
243 suppliers
$12.00/5g

939412-86-9
135 suppliers
$9.00/250mg
Yield:939412-86-9 153.5 g
Reaction Conditions:
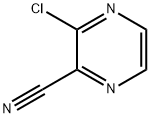
Stage #1:2-chloro-3-cyanopyrazine with hydrogen;acetic acid in water at 20; under 3000.3 Torr;
Stage #2: with hydrogenchloride in diethyl ether;ethyl acetate at 20;Cooling with ice;
Steps:
a (a) (3-Chloropyrazin-2-yl)methanamine. hydrochloride
To a solution of 3-chloropyrazine-2-carbonitrile (160 g, 1.147 mol) in acetic acid (1.5 L) was added Raney Nickel (50% slurry in water, 70 g, 409 mmol). The resulting mixture was stirred under 4 bar hydrogen at room temperature overnight. Raney Nickel was removed by filtration over decalite and the filtrate was concentrated under reduced pressure and co-evaporated with toluene. The remaining brown solid was dissolved in ethyl acetate at 50°C and cooled on an ice-bath. 2M hydrogen chloride solution in diethyl ether (1.14 L) was added in 30 min. The mixture was allowed to stir at room temperature over weekend. The crystals were collected by filtration, washed with diethyl ether and dried under reduced pressure at 40°C. The product brown solid obtained was dissolved in methanol at 60°C. The mixture was filtered and partially concentrated, cooled to room temperature and diethyl ether (1000 ml) was added. The mixture was allowed to stir at room temperature overnight. The solids formed were collected by filtration, washed with diethyl ether and dried under reduced pressure at 40°C to give 153.5 g of (3-chloropyrazin-2-yl)methanamine. hydrochloride as a brown solid (74.4 %, content 77 %).
References:
MSD OSS B.V.;MAN, de Adrianus, Petrus, Antonius;WIJKMANS, Jacobus C.H.M.;STERRENBURG, Jan-Gerard;RAAIJMAKERS, Hans C.A.;BARF, Tjeerd A.;BUIJSMAN, Rogier, Christian;OUBRIE, Arthur A.;REWINKEL, Johannes, Bernardus, Maria;JANS, Christiaan, Gerardus, Johannes, Maria;STOCK, Herman, Thijs WO2013/10869, 2013, A1 Location in patent:Page/Page column 25

867165-55-7
12 suppliers
inquiry

939412-86-9
135 suppliers
$9.00/250mg