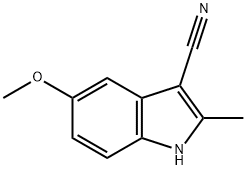
3-CYANO-5-METHOXY-2-METHYLINDOLE synthesis
- Product Name:3-CYANO-5-METHOXY-2-METHYLINDOLE
- CAS Number:481668-37-5
- Molecular formula:C11H10N2O
- Molecular Weight:186.21

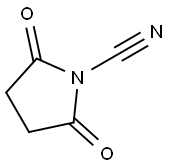
6260-86-2
47 suppliers
$253.75/1g

481668-37-5
13 suppliers
inquiry
Yield: 50%
Reaction Conditions:
with aluminum (III) chloride;sodium azide in tetrahydrofuran for 8 h;Reflux;
Steps:
Typical procedure for the preparation of substituted 2-methylindole-3-carbonitriles (8a-8h)
General procedure: To a solution of AlCl3 (2.67 g, 20.0 mmol) in THF (100 mL), NaN3 (3.90 g, 60.0 mmol) and corresponding substituted 2-methylindole-3-carboxadehyde 7 (10.0 mmol) were added. The reaction mixture was heated at reflux for 8 hours. The solvent was then removed under reduced pressure, and the residue was diluted with 10% HCl (20 mL). This mixture was extracted with CH2Cl2 (2 X 60 mL). The combined organic layer was dried over Na2SO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (EtOAc/hexanes = 1:3) to obtain the corresponding carbonitrile.
References:
Thanetchaiyakup, Adisak;Rattanarat, Hassayaporn;Chuanopparat, Nutthawat;Ngernmeesri, Paiboon [Tetrahedron Letters,2018,vol. 59,# 11,p. 1014 - 1018] Location in patent:supporting information
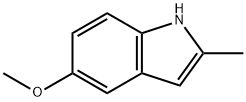
1076-74-0
194 suppliers
$15.00/1g
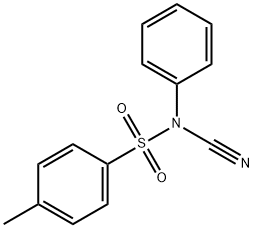
55305-43-6
66 suppliers
$23.00/100mg

481668-37-5
13 suppliers
inquiry

89302-15-8
35 suppliers
inquiry

481668-37-5
13 suppliers
inquiry

1076-74-0
194 suppliers
$15.00/1g

481668-37-5
13 suppliers
inquiry