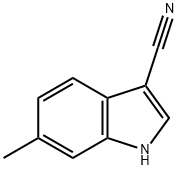
3-CYANO-6-METHYLINDOLE synthesis
- Product Name:3-CYANO-6-METHYLINDOLE
- CAS Number:194490-23-8
- Molecular formula:C10H8N2
- Molecular Weight:156.18
4771-49-7
135 suppliers
$11.00/250mg

194490-23-8
14 suppliers
inquiry
Yield:194490-23-8 76%
Reaction Conditions:
with formic acid;hydroxylamine hydrochloride;sodium formate for 1 h;Heating / reflux;
Steps:
4.1.1; 4.3.1
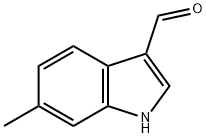
Example 4; 1. Synthesis of indole derivatives; 1) Synthesis of 3-cyanoindole derivatives; [Show Image] Nine objective compounds were prepared by (1) formylation of 3-position of the corresponding indole using phosphorus oxychloride in the presence of dimethylformamide (Vilsmeier method), (2) cyanation by dehydrating reaction with hydroxylamine in sodium formate and formic acid, (3) coupling with ethyl 4-fluorobenzoate in the presence of potassium fluoride on almina and 18-crown-6-ether in dimethyl sulfoxide and then, (4) hydrolysis with lithium hydroxide in total 4 steps in that order (the following Table 2). In addition, XO-CH172 and XO-CH183 (R is a 2-methyl group or a 5-methoxy group, respectively) were prepared from the step (2) using the corresponding aldehydes purchased.; XO-CH186; XO-CH180 (1.14g, 7.16 mmol) was dissolved in formic acid (11 mL), to the solution were added hydroxylamine hydrochloride (0.63 g, 9.1 mmol) and sodium formate (0.90 g, 13 mmol). The mixture was heated for reflux for an hour. To the reaction mixture was added water under ice-cooling, and the mixture was stirred for a while. The solid was collected by filtration and dried 60°C under reduced pressure to give XO-CH186 as a red-black solid (0.85 g, 76% yield).
References:
EP1932833,2008,A1 Location in patent:Page/Page column 15; 19