
3-cyano-N-methylbenzenesulfonamide synthesis
- Product Name:3-cyano-N-methylbenzenesulfonamide
- CAS Number:56542-62-2
- Molecular formula:C8H8N2O2S
- Molecular Weight:196.23
Yield:56542-62-2 99%
Reaction Conditions:
in dichloromethane;water at 0; for 0.5 h;
Steps:
97.1
Example 97; 2-(3-Fluoro-phenyl)-pyrimidine-5-carboxylic acid 3-methylsulfamoyl-benzylamide; Step 1; A flask containing DCM (90 niL) and methylamine in water (40 wt%, 7.5 mmol) is chilled in an ice water bath with magnetic stirring: 3-Cyano-benzenesulfonyl chloride (5 g, 2.49 mmol) is added along with DCM (1OmL) to wash down the sides of the flask. After 30 min, concentrated HCl in water is added at O0C, until the reaction is acidic (pH < 4). Water (50 mL) is added and DCM is removed in vacuo. The residue is filtered to afford 3-cyano-N-methyl-benzenesulfonamide as a solid (99%). MS: 195 (M+H); 1HNMR (300 MHz, CDCl3): δ 2.76 (d, 3H), 4.48 (broad-s, N-H), 7.7 (t, IH), 7.9 (d, IH), 8.13 (d, IH), 8.19 (s, IH).
References:
WO2007/41634,2007,A1 Location in patent:Page/Page column 75
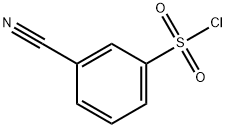
56542-67-7
154 suppliers
$14.00/1g

593-51-1
529 suppliers
$21.00/100g

56542-62-2
11 suppliers
inquiry

56542-67-7
154 suppliers
$14.00/1g

56542-62-2
11 suppliers
inquiry
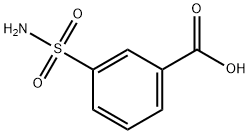
636-76-0
87 suppliers
$10.00/100mg

56542-62-2
11 suppliers
inquiry
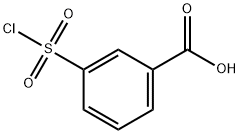
4025-64-3
207 suppliers
$6.00/1g

56542-62-2
11 suppliers
inquiry
