
3-Cycloocten-1-ol synthesis
- Product Name:3-Cycloocten-1-ol
- CAS Number:4114-99-2
- Molecular formula:
- Molecular Weight:0
![9-Oxabicyclo[6.1.0]non-2-ene, (1R-cis)- (9CI)](/CAS/20210305/GIF/183904-03-2.gif)
183904-03-2
0 suppliers
inquiry

4114-99-2
0 suppliers
inquiry
Yield:4114-99-2 77%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20;Inert atmosphere;
Steps:
4
A solution of LiAlH4 (0.5 eq., 81.5 ml of a 1.0 M solution in THF, 81.5 mmol) was added dropwise at 0° C. and under Ar to a stirred solution of 17 (1.0 eq., 20.23 g, 163 mmol) in THF (1.0 M, 163 ml). The reaction mixture was allowed to warm up to rt and stirred o/n. H2O (about 20 ml) was carefully added to stop the reaction. The reaction mixture was filtered, dried over Na2SO4, and concentrated. FC (20% EtOAc in C6H12) yielded 18 (15.81 g, 125 mmol, 77%) as a clear liquid. (0803) Rf (20% EtOAc in C6H12)=0.4. (0804) 1H-NMR (CDCl3) δ=5.76-5.60 (m, 2H), 3.84-3.77 (m, 1H), 2.36 (dd, 3J(H,H)=7.5, 6.3 Hz, 2H), 2.28-2.18 (m, 1H), 2.14-2.05 (m, 1H), 1.87-1.78 (m, 1H), 1.73-1.63 (m, 1H), 1.61-1.43 (m, 4H), 1.40-1.30 (m, 1 H) ppm. (0805) 13C-NMR (CDCl3) δ=132.4, 125.0, 72.2, 35.1, 34.0, 28.3, 25.7, 21.2 ppm.
References:
US2016/340297,2016,A1 Location in patent:Paragraph 0802-0805
![9-oxabicyclo[6.1.0]non-2-ene](/CAS/GIF/6690-12-6.gif)
6690-12-6
1 suppliers
inquiry

4114-99-2
0 suppliers
inquiry
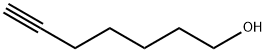
63478-76-2
157 suppliers
$9.00/1g

4114-99-2
0 suppliers
inquiry

14916-79-1
95 suppliers
$40.21/5g

4114-99-2
0 suppliers
inquiry
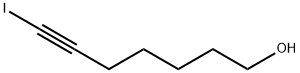
164470-73-9
1 suppliers
inquiry

4114-99-2
0 suppliers
inquiry