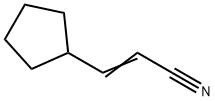
3-CYCLOPENTYLACRYLONITRILE synthesis
- Product Name:3-CYCLOPENTYLACRYLONITRILE
- CAS Number:591769-05-0
- Molecular formula:C8H11N
- Molecular Weight:121.18
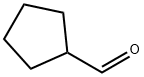
872-53-7
266 suppliers
$9.00/1g
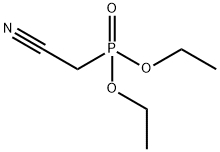
2537-48-6
327 suppliers
$7.00/10g

591769-05-0
215 suppliers
$11.00/1g
Yield:591769-05-0 89%
Reaction Conditions:
Stage #1: diethyl 1-cyanomethylphosphonatewith potassium tert-butylate in tetrahydrofuran at 0 - 20;
Stage #2: cyclopentanealdehyde in tetrahydrofuran at 0 - 20; for 64 h;
Steps:
67.1 Step 1. (2E)- and (2Z)-3-Cyclopentylacrylonitrile
Step 1.
(2E)- and (2Z)-3-Cyclopentylacrylonitrile
To a solution of 1.0 M potassium tert-butoxide in THF (235 mL) at 0 °C was added dropwise a solution of diethyl cyanomethylphosphonate (39.9 mL, 0.246 mol) in THF (300 mL).
The cold bath was removed and the reaction was warmed to room temperature followed by recooling to 0 °C, at which time a solution of cyclopentanecarbaldehyde (22.0 g, 0.224 mol) in THF (60 mL) was added dropwise.
The bath was removed and the reaction warmed to ambient temperature and stirred for 64 hours.
The mixture was partitioned between diethyl ether and water, the aqueous was extracted with three portions of ether, followed by two portions of ethyl acetate.
The combined extracts were washed with brine, then dried over sodium sulfate, filtered and concentrated in vacuo to afford a mixture containing 24.4 g of olefin isomers which was used without further purification (89%).
1H NMR (400 MHz, CDCl3): δ 6.69 (dd, 1H, trans olefin), 6.37 (t, 1H, cis olefin), 5.29 (dd, 1H, trans olefin), 5.20 (d, 1H, cis olefin), 3.07-2.95 (m, 1H, cis product), 2.64-2.52 (m, 1H, trans product), 1.98-1.26 (m, 16H).
References:
EP2349260,2016,B1 Location in patent:Paragraph 0111

872-53-7
266 suppliers
$9.00/1g
15898-47-2
44 suppliers
$10.00/100mg

591769-05-0
215 suppliers
$11.00/1g
112-62-9
467 suppliers
$6.00/25g

107-13-1
399 suppliers
$17.00/25ML

591769-05-0
215 suppliers
$11.00/1g