
3-cyclopropyl-1,2,4-thiadiazol-5-amine synthesis
- Product Name:3-cyclopropyl-1,2,4-thiadiazol-5-amine
- CAS Number:762272-35-5
- Molecular formula:C5H7N3S
- Molecular Weight:141.19
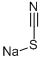
540-72-7
422 suppliers
$13.50/100G
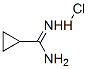
57297-29-7
205 suppliers
$8.00/1g

762272-35-5
28 suppliers
$200.00/25mg
Yield:762272-35-5 243 mg
Reaction Conditions:
Stage #1: sodium thiocyanide;cyclopropylcarboxamidine hydrochloridewith triethylamine in methanol at -20; for 0.75 h;
Stage #2: with sodium hypochlorite;triethylamine in methanol at -20 - 20; for 14 h;
Steps:
49.1 3-Cyclopropyl-1,2,4-thiadiazol-5-amine
Step 1
3-Cyclopropyl-1,2,4-thiadiazol-5-amine
Sodium thiocyanate (527 mg, 6.50 mmol) was dissolved in 20 mL of methanol and placed in -20°C, followed by addition of cyclopropylcarbamidine hydrochloride (603 mg, 5 mmol) and triethylamine (0.8 mL, 5.74 mmol).
After stirring for 45 minutes, triethylamine (0.7 mL, 5.02 mmol) and 8% sodium hypochlorite solution(4.2 mL, 5 mmol) were dropwise added into the reaction mixture.
After reacting for 2 hours at -20°C, the reaction mixture was warmed up to room temperature.
After reacting for 12 hours, the reaction mixture was concentrated under reduced pressure, followed by addition of 35 mL of H2O and extracted with ethyl acetate (30 mL*3).
The organic phase was combined, washed with saturated sodium chloride solution (50 mL), dried over anhydrous sodium sulfate and filtered.
The filtrate was concentrated under reduced pressure to obtain the crude title product 3-cyclopropyl-1,2,4-thiadiazol-5-amine 49a (243 mg, white solid), which was used directly in the next step without further purification. MS m/z (ESI): 142.2 [M+1]
References:
EP2796460,2014,A1 Location in patent:Paragraph 0240