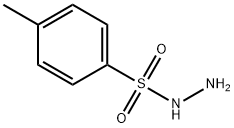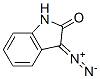
3-Diazo-2,3-dihydro-1H-indole-2-one synthesis
- Product Name:3-Diazo-2,3-dihydro-1H-indole-2-one
- CAS Number:3265-29-0
- Molecular formula:
- Molecular Weight:0
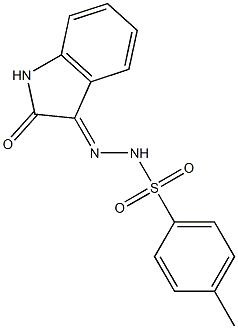
28558-62-5
0 suppliers
inquiry

3265-29-0
1 suppliers
inquiry
Yield:3265-29-0 96%
Reaction Conditions:
with sodium hydroxide in water at 50; for 3 h;
Steps:
2. step:
General procedure: The corresponding isatin p-tosylhydrazone (n; mmol) was suspended in water (10 mL per each1 mmol of p-tosylhydrazone) and 10% aqueous NaOH (4.5 mmol per each 1 mmol oftosylhyrazone; 4.5 equiv) was added in one portion. The suspension was stirred at 50 °C untildissolution of all solid material accompanied by a change of the solution color to orange (theating).Then, the reaction mixture was cooled and extracted with EtOAc (4 × 75 mL). The combinedorganic layers were washed with water (50 mL), dried with anhydrous Na2SO4, and evaporated.Yields and 1H NMR data are given below.
References:
Marek, Luká?;Kolman, Luká?;Váňa, Ji?í;Svoboda, Jan;Hanusek, Ji?í [Beilstein Journal of Organic Chemistry,2021,vol. 17,p. 527 - 539] Location in patent:supporting information
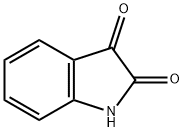
91-56-5
437 suppliers
$5.00/25g

3265-29-0
1 suppliers
inquiry
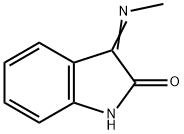
2058-70-0
2 suppliers
inquiry

3265-29-0
1 suppliers
inquiry

28558-62-5
0 suppliers
inquiry

3265-29-0
1 suppliers
inquiry