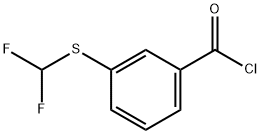
3-(DIFLUOROMETHYLTHIO)BENZOYL CHLORIDE synthesis
- Product Name:3-(DIFLUOROMETHYLTHIO)BENZOYL CHLORIDE
- CAS Number:261944-16-5
- Molecular formula:C8H5ClF2OS
- Molecular Weight:222.64
4837-24-5
9 suppliers
inquiry

261944-16-5
26 suppliers
$144.00/1g
Yield:-
Reaction Conditions:
with thionyl chloride in toluene at 100; for 1 h;
Steps:
1 Reference Example 1:
To a dioxane (150 mL) solution of 3-mercaptobenzoic acid (25.0 g, 0.162 mol) were added water (50 mL) and sodium hydroxide (40 g, 1.0 mol), followed by heating under stirring at 60°C. Therein was bubbled Flon 22 (difluorochloromethane) gas for 9 hours. Fron 22 was bubbled in at such a rate that it was gently refluxed by means of a gas trap cooled with dry ice-acetone, and during the time, each 10 g of sodium hydroxide was further added twice. After completion of the reaction, dioxane was removed by evaporation and then the residue was acidified by adding concentrated hydrochloric acid. The resulting residue was dissolved in ethyl acetate and insoluble matter was filtrated through Celite (trade name). Thereafter, the filtrate was washed with water and brine and then the solvent was removed by evaporation. Based on NMR inspection of the residue, the ratio of 3-(difluoromethylthio)benzoic acid to the starting material was found to be about 9:1. Subsequently, thionyl chloride (28.4 g, 0.239 mol) and toluene (30 mL) were added to crude 3-(difluoromethylthio)benzoic acid (32.5 g) obtained above, followed by heating under stirring at 100°C for 1 hour. After completion of the reaction, the solvent was removed by evaporation to obtain a crude acid chloride. Then, the acid chloride obtained above was added to a mixed solution of ethanol (100 mL) and triethylamine (24.1 g, 0.239 mol) cooled with ice-water, followed by stirring at room temperature for 3 hours. After completion of the reaction, the solvent was removed by evaporation and the obtained residue was dissolved in ethyl acetate. The solution was washed with water and brine and then the solvent was removed by evaporation. The residue was purified on a silica gel column (Kiesel gel 60 manufactured by MERCK, 2% AcOEt-Hex) to obtain ethyl 3-(difluoromethylthio)benzoate (30.7 g, 84%).1H-NMR (400MHz, CDCl3) : 1.41(t, 3H, J=7.2Hz), 4.40(q, 2H, J=7.2Hz), 6.86(t, 1H, J=56.8Hz), 7.48(t, 1H, J=8.0Hz), 7.77(dt, 1H, J=8.0Hz, 1.6Hz), 8.10(dt, 1H, J=8.0Hz, 1.6Hz), 8.25(t, 1H, J=1.6Hz).
References:
EP1544202,2005,A1 Location in patent:Page/Page column 50-51