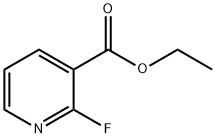
3-(ethoxycarbonyl)-2-fluoropyridine synthesis
- Product Name:3-(ethoxycarbonyl)-2-fluoropyridine
- CAS Number:113898-56-9
- Molecular formula:C8H8FNO2
- Molecular Weight:169.15
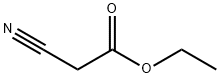
105-56-6
462 suppliers
$10.00/5ml
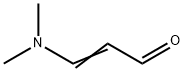
927-63-9
205 suppliers
$14.00/1g

113898-56-9
35 suppliers
$85.00/250mg
Yield:113898-56-9 90.4%
Reaction Conditions:
Stage #1: ethyl 2-cyanoacetate;3-dimethylaminoacroleinwith 1-butyl-3-methylimidazolium trifluoroacetate at 120; for 2 h;
Stage #2: with hydrogen fluoride
Steps:
6 Example 6 Synthesis of ethyl 2-fluoronicotinate
In a reactor, 59 mL (0.5 mol) of cyanoacetic acid ethyl ester was added,1-butyl-3-methylImidazole trifluoroAcetate50 mL,3-dimethylaminopropenal 62 mL (0.5 mol) was homogeneously mixed,Electric furnace sets heated to 120 temperature and heat 2h reaction,TLC detection (petroleum ether: methylene chloride 1: 2 expansion, sublimation iodine color) 3-dimethylaminopropenal reaction completely, cooled to room temperature, organic solvent extraction of 60mL of toluene 3 times,The residual phase was washed with water and dried in vacuo. The organic phase was passed through a dry HF gas and the reaction was followed by HPLC until the reaction was complete. Add the mass fraction of 20% potassium carbonate solution to adjust the pH = 5-6, liquid, water layerWith toluene 20mL × 3 times extraction,The organic layers were combined, washed with water, separated by molecular sieves, filtered, and the solvent was distilled off under reduced pressure.The yield of ethyl 2-fluoronicotinate and 76.5 g of light brown liquid was 90.4%.
References:
CN105001154,2017,B Location in patent:Paragraph 0057; 0058; 0059

105-56-6
462 suppliers
$10.00/5ml
13070-22-9
2 suppliers
inquiry

113898-56-9
35 suppliers
$85.00/250mg

614-18-6
478 suppliers
$5.00/10g

113898-56-9
35 suppliers
$85.00/250mg

105-56-6
462 suppliers
$10.00/5ml

113898-56-9
35 suppliers
$85.00/250mg
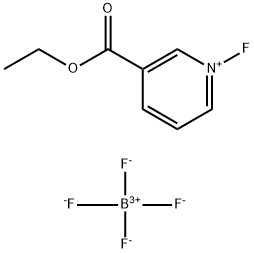
116241-52-2
0 suppliers
inquiry

113898-56-9
35 suppliers
$85.00/250mg

116241-59-9
18 suppliers
$45.00/10mg