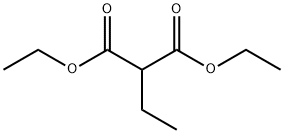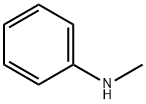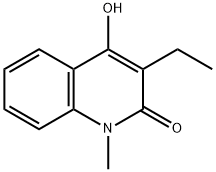
3-Ethyl-4-hydroxy-1-Methylquinolin-2(1H)-one synthesis
- Product Name:3-Ethyl-4-hydroxy-1-Methylquinolin-2(1H)-one
- CAS Number:14944-99-1
- Molecular formula:C12H13NO2
- Molecular Weight:203.24
Yield:14944-99-1 89%
Reaction Conditions:
at 220 - 270;
Steps:
4.2 General procedure for the preparation of 4-hydroxyquinolin-2(1H)-ones (1)
General procedure: A mixture of the appropriate aniline (100mmol) and substituted diethyl malonate (102mmol) was heated in a flask equipped with distillation head on a metal bath at 220-230°C for 1h and then at 260-270°C until the distillation of ethanol stopped (3-6h). With the exception of preparation of 1f, l, m, the hot liquid reaction mixture was carefully poured into stirred toluene (50mL), cooled down to room temperature and the precipitate was collected by filtration. In the case of 1f, l, m, the hot reaction mixture solidified and it was cooled down to room temperature. The above precipitate or solidified material was mixed with aqueous sodium hydroxide solution (0.5M, 250mL) and toluene (50mL). The substance that remained undissolved (in the preparation of 1c and 1m) was removed by filtration, purified by recrystallization and identified as propanediamide derivatives (1c′ and 1m′, respectively). The layers of the filtrate were separated and the aqueous layer was washed with toluene (2×40mL). The water layer was treated with decolorizing charcoal, filtered and then acidified with 10% HCl to Congo red. The precipitated hydroxyquinolone 1 was collected by filtration, washed with water, and if necessary, purified by recrystallization.
References:
Kafka, Stanislav;Proisl, Karel;Ka?párková, Věra;Urankar, Damijana;Kimmel, Roman;Ko?mrlj, Janez [Tetrahedron,2013,vol. 69,# 51,p. 10826 - 10835]

54289-76-8
13 suppliers
inquiry

14944-99-1
6 suppliers
inquiry
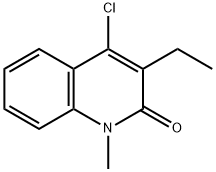
110254-72-3
0 suppliers
inquiry

14944-99-1
6 suppliers
inquiry