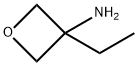
3-ethyl-oxetan-3-ylaMine synthesis
- Product Name:3-ethyl-oxetan-3-ylaMine
- CAS Number:1363383-14-5
- Molecular formula:C5H11NO
- Molecular Weight:101.15
1365969-56-7
17 suppliers
$501.88/5MG

1363383-14-5
15 suppliers
inquiry
Yield:1363383-14-5 860 mg
Reaction Conditions:
with cyclohexa-1,4-diene;palladium 10% on activated carbon;hydrogen in methanol at 25; for 4 h;Inert atmosphere;
Steps:
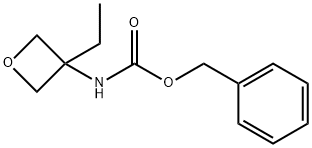
Synthesis of 3-ethyloxetan-3-amine
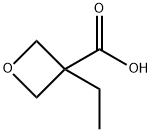
Synthesis of 3-ethyloxetan-3-amine: 3-ethyloxetane-3- carboxylic acid (3.0g, 23.1 mmol), DPPA (Diphenylphosphoryl azide, 7.61 g, 27.7 mmol), triethylamine (3.0 g, 23.1 mmol) and BnOH (2.99 g, 27.7 mmol) were dissolved in toluene (50 mL). The mixture was stirred at 110°C overnight. The solvent was removed in vacuo. Dichloromethane (50 mL) was added. The mixture was washed with IN HC1 (20 mL). The aqueous layer was extracted with dichloromethane (20 mL). The combined organic layers were washed with brine and dried over Na2S04. The solvent was removed in vacuo. The residue was purified by column chromatography over silica gel (eluent: petroleum ether / ethyl acetate from 100/1 to 60/40) resulting in benzyl 3-ethyloxetan-3-ylcarbamate (4.0 g). To a solution of benzyl 3-ethyloxetan-3-yl- carbamate (2.0g, 8.5mmol) and cyclohexa-1, 4-diene (1.02 g, 12.75 mmol) in MeOH (20 mL) was added Pd-C (10%, 0.2 g) under N2. The mixture was stirred under H2 balloon at 25°C for 4 hours. After filtration, the filtrate was concentrated resulting in 3-ethyloxetan-3-amine (860 mg), which was used as such in the next reaction.
References:
WO2014/33176,2014,A1 Location in patent:Page/Page column 36
28562-61-0
63 suppliers
$75.00/250mg

1363383-14-5
15 suppliers
inquiry