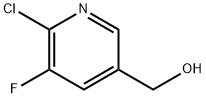
3-Fluoro-5-(hydroxymethyl)-2-methylpyridine synthesis
- Product Name:3-Fluoro-5-(hydroxymethyl)-2-methylpyridine
- CAS Number:917835-69-9
- Molecular formula:C7H8FNO
- Molecular Weight:141.14
Yield:917835-69-9 39%
Reaction Conditions:
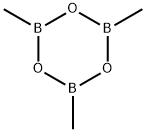
with potassium carbonate;tetrakis(triphenylphosphine) palladium(0) in 1,4-dioxane at 110; for 20 h;
Steps:
5.c
(c) (5-Fluoro-6-methyl-3-pyridinyl)methanolTo a solution of (6-chloro-5-fluoro-3-pyridinyl)methanol (1.3 g, 8.05 mmol) in 1,4-dioxane (10 ml) was added K2CO3 (3.34 g, 24.14 mmol), trimethylboroxin (1.125 ml, 8.05 mmol) and Pd(PPh3)4 (0.465 g, 0.402 mmol). The mixture was then heated at 110° C. for 20 h in a pressure tube. The resulting mixture was quenched with H2O, extracted with EtOAc, dried over MgSO4 and concentrated under vacuum. The residue was purified by flash chromatography using Flashmaster II, a 25 g spherical silica gel cartridge and DCM/MeOH 98:2 as eluent to give 560 mg of the title compound along with Ph3PO. This was washed again with water and extracted with EtOAc to remove Ph3PO. The organic layer was dried over MgSO4, filtered and concentrated under vacuum to give 468 mg (39%) of the title compound pure enough to be used in the next step.1H-NMR (δ, ppm, CDCl3): 8.27 (d, 1H), 7.55 (dd, 1H), 4.74 (bs, 2H), 2.49 (s, 3H). [ES MS] m/z 142 (MH+).
References:
US2009/306089,2009,A1 Location in patent:Page/Page column 27

82671-03-2
57 suppliers
$86.00/1g

917835-69-9
23 suppliers
inquiry
82671-06-5
306 suppliers
$5.00/1g

917835-69-9
23 suppliers
inquiry