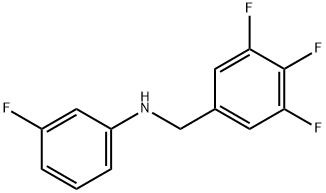
3-fluoro-N-(3,4,5-trifluorobenzyl)aniline synthesis
- Product Name:3-fluoro-N-(3,4,5-trifluorobenzyl)aniline
- CAS Number:637744-49-1
- Molecular formula:C13H9F4N
- Molecular Weight:255.21
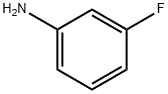
372-19-0
324 suppliers
$10.00/1g
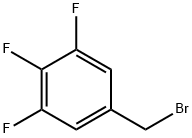
220141-72-0
127 suppliers
$13.00/1g

637744-49-1
13 suppliers
$243.00/250mg
Yield:637744-49-1 84.8%
Reaction Conditions:
with potassium carbonate in toluene at 20; for 21 h;Heating / reflux;
Steps:
16 Preparation 16; Intermediate I-8 - preparation of [(3-FLUOROPHENYL)- (3,] 4, 5-trifluorobenzyl) amine
A mixture of 3.7 g (3.2 ml, 33.3 [MMOL)] of 3-fluorophenylamine, 2. 5 g (11.1 [MMOL)] of 5- (bromomethyl)-1, 2, 3-trifluorobenzene and 1. 53 g [(11. 1 MMOL) OF K2CO3 IN 30 ML OF] toluene, was refluxed during 5 h and stirred at room temperature during 16 hours more. After this time the mixture of reaction was filtered and the solid obtained was washed with toluene. The toluene solutions were combined, washed with water and brine, dried over [MGSO4,] and concentrated in vacuo to dryness to give 5.0 g of an oily residue. This oil was treated with diethyl ether and the obtained solid was separated by filtration and discarded. The filtrate was concentrated to dryness and purified by Kugelrohr distillation at reduced pressure. After distillation of the excess of [3-FLUOROPHENYLAMINE] (0.15 mm Hg, [100C] oven), 2.40 g (84.8%) of the title. product were distilled (0.15 mm Hg, [175-200C] oven). Structure confirmed by MS and'H- RMN. GC/MS: [M] + : 255 [1H-NMR (CDC13)] : 5 4.30 (s, 2H), 4.0-4. 50 (bs, [1H),] 6.20-6. 55 (m, 3H), 6.80-7. 25 (m, 3H).
References:
WO2004/840,2003,A2 Location in patent:Page 27
1202493-05-7
11 suppliers
$341.00/1g

637744-49-1
13 suppliers
$243.00/250mg
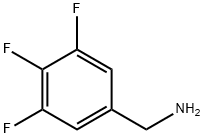
235088-69-4
108 suppliers
$8.00/250mg
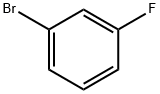
1073-06-9
428 suppliers
$6.00/10g

637744-49-1
13 suppliers
$243.00/250mg

372-19-0
324 suppliers
$10.00/1g

637744-49-1
13 suppliers
$243.00/250mg