
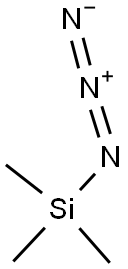
3-fluorobenzyl azide synthesis
- Product Name:3-fluorobenzyl azide
- CAS Number:159979-97-2
- Molecular formula:C7H6FN3
- Molecular Weight:151.14
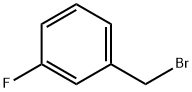
456-41-7
288 suppliers
$13.00/25g

159979-97-2
16 suppliers
inquiry
Yield:159979-97-2 99.7%
Reaction Conditions:
with Caswell No. 744A in lithium hydroxide monohydrate;tert-butyl alcohol;
Steps:
4.1. General Procedure for the Synthesis of Organic Azides
General procedure: Into a 250 mL round-bottom ask, organic bromide (10 mmol), tert-butyl alcohol/water (1:1,100 mL), and sodium azide (0.98 g, 15 mmol) were added in that order. The mixture was stirred at rtfor 2±3 h. The mixture was extracted with ethyl acetate (3 25 mL). The organic layers were combinedand washed with brine, dried over anhydrous sodium sulfate, and evaporated in vacuo to dryness.Air was gently passed over the oily product to remove traces of ethyl acetate, affording the organicazide in 80-95% yield. The azide was then stored at 0 C and used directly without further purification.
References:
Trujillo, Marissa;Hull-Crew, Clayton;Outlaw, Andrew;Stewart, Kevin;Taylor, Loren;George, Laura;Duensing, Allison;Tracey, Breanna;Schoffstall, Allen [Molecules,2019,vol. 24,# 5,art. no. 973;]
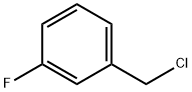
456-42-8
331 suppliers
$5.00/5g

159979-97-2
16 suppliers
inquiry

352-70-5
249 suppliers
$14.00/5g

159979-97-2
16 suppliers
inquiry
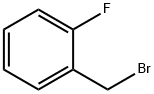
446-48-0
334 suppliers
$6.00/10g

159979-97-2
16 suppliers
inquiry
4648-54-8
295 suppliers
$16.80/5G

456-41-7
288 suppliers
$13.00/25g

159979-97-2
16 suppliers
inquiry