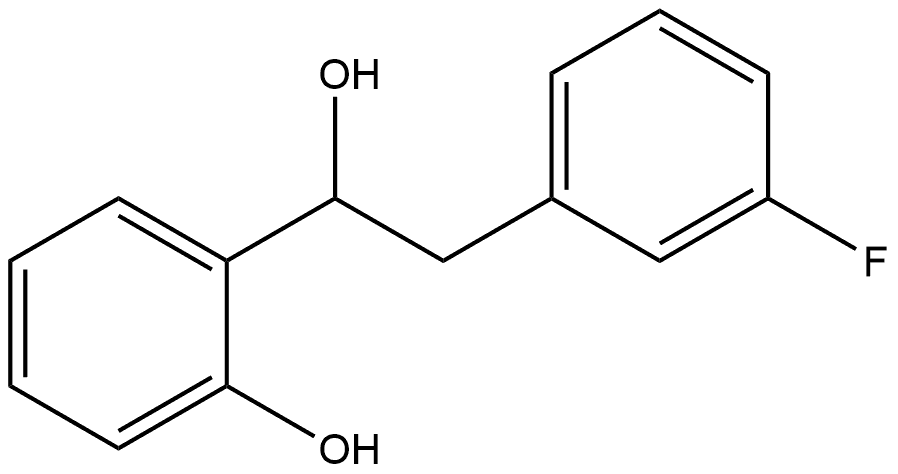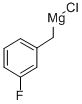
3-FLUOROBENZYLMAGNESIUM CHLORIDE synthesis
- Product Name:3-FLUOROBENZYLMAGNESIUM CHLORIDE
- CAS Number:64168-34-9
- Molecular formula:C7H6ClFMg
- Molecular Weight:168.88

79099-07-3
543 suppliers
$5.00/5g

64168-34-9
15 suppliers
$210.00/100ml
![1-Piperidinecarboxylic acid, 4-[(3-fluorophenyl)methyl]-4-hydroxy-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1026027-59-7.gif)
1026027-59-7
0 suppliers
inquiry
Yield:1026027-59-7 42%
Reaction Conditions:
in tetrahydrofuran at 25; for 16 h;Inert atmosphere;
Steps:
76.1 Step 1: Preparation of tert-butyl 4-(3-fluorobenzyl)-4-hydroxypiperidine-1-carboxylate
To a solution of (3-fluorobenzyl)magnesium chloride (0.5 M in tetrahydrofuran, 12 mL, 6 mmol) in dry tetrahydrofuran (20 mL) at 0 °C, was added a solution of tert-butyl 4-oxopiperidine-1-carboxylate (1 g, 5 mmol) in dry tetrahydrofuran (10 mL) dropwise under argon. The reaction mixture was stirred at 25 C for 16 h. The reaction was quenched with aqueous ammonium chloride solution, diluted with ethyl acetate/water (20 mL/20 mL) and extracted with ethyl acetate (30 mL x 2). The combined organic layers were washed with brine (50 mL), dried over sodium sulfate, filtered and concentrated under reduced pressure. The crude residue was purified by Combi-Flash (Biotage, 40 g silica gel, eluted with ethyl acetate in petroleum ether from 30% to 40%) to give tert-butyl 4-(3-fluorobenzyl)-4-hydroxypiperidine-1- carboxylate (0.65 g, 2.1 mmol, 42%) as a colorless oil.1H NMR (400 MHz, Chloroform-d) d 7.28-7.35 (m, 1H), 6.90-7.03 (m, 3H), 3.78-4.04 (m, 2H), 3.02-3.21 (m, 2H), 2.77 (s, 2H), 1.41-1.54 (m, 13H); LCMS (ESI) m/z: 236.1 [M-56+H]+.
References:
WO2020/154571,2020,A1 Location in patent:Page/Page column 162
91830-10-3
0 suppliers
inquiry

64168-34-9
15 suppliers
$210.00/100ml
![2-Pyrrolidinone, 5-[4-(3-fluorophenyl)-3-oxobutyl]-](/CAS/20210305/GIF/346673-02-7.gif)
346673-02-7
0 suppliers
inquiry

91830-10-3
0 suppliers
inquiry
431988-79-3
0 suppliers
inquiry

64168-34-9
15 suppliers
$210.00/100ml
![2-Pyrrolidinone, 5-[4-(3-fluorophenyl)-3-oxobutyl]-](/CAS/20210305/GIF/346673-02-7.gif)
346673-02-7
0 suppliers
inquiry

64168-34-9
15 suppliers
$210.00/100ml
![Benzene, 1-(4-bromobutoxy)-2-[2-(3-fluorophenyl)ethenyl]-, (E)- (9CI)](/CAS/20210305/GIF/89122-81-6.gif)
89122-81-6
0 suppliers
inquiry