
3-Hexanol, 1-(phenylmethoxy)- synthesis
- Product Name:3-Hexanol, 1-(phenylmethoxy)-
- CAS Number:140654-19-9
- Molecular formula:C13H20O2
- Molecular Weight:208.3
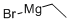
925-90-6
346 suppliers
$12.00/5ml
![Oxirane, 2-[2-(phenylMethoxy)ethyl]-](/CAS/GIF/94426-72-9.gif)
94426-72-9
32 suppliers
$65.00/100mg

140654-19-9
0 suppliers
inquiry
Yield:140654-19-9 97%
Reaction Conditions:
Stage #1: ethylmagnesium bromidewith copper(l) iodide in tetrahydrofuran at 0; for 0.5 h;
Stage #2: 2-(2-(benzyloxy)ethyl)oxirane in tetrahydrofuran at 0 - 25; for 1 h;
Steps:
B Step B: (R or S ) -1- (benzyloxy) hexan-3-ol
Set up a reactor R-1 with an agitator. (Note: the reactor R-1: 1 L bottle) , Charged EtMgBr (150 mL, 3.75 X by volume) into reactor R-1. Charged THF (240 mL, 6.00 X by volume) into reactor R-1. Charged CuI (0.85 g, 0.02 X by weight) into reactor R-1 at 0 oC. Stired the mixture at 0 for 0.5 h. Charged (R or S) -2- (2- (benzyloxy) ethyl) oxirane (40.0 g, 1.00 X by weight) into reactor R-1. Stired the mixture at 25 for 1 h. TLC (petroleum ether/ethyl acetate =3/1, Rf= 0.35) showed the reaction was completed. Added aq. NH4Cl (200 mL, 5.00 X by volume) to the mixture. Extracted the mixture with EtOAc (200 mL x 2, 10.0 X by volume) . Combined the organic layer and washed the organic layer with brine. Dried the organic layer with Na2SO4. Concentrated the organic layer to get the crude product. The residue was purified by column chromatography (SiO2, petroleum ether/ethyl acetate = 50/1 to 5/1) . Target (22.7 g, 47.1%yield, 97.0%purity) as a yellow oil .1HNMR: 400 MHz CDCl3:δ 7.30 -7.38 (m, 5H) , 4.54 (s, 2H) , 3.74 -3.83 (m, 1H) , 3.69 -3.73 (m, 1H) , 3.65 -3.67 (m, 1H) , 2.87 (s, 1H) , 1.75 -1.77 (m, 2H) , 1.36 -1.48 (m, 4H) , 0.92 -0.95 (m, 3H) ppm.
References:
WO2020/160711,2020,A1 Location in patent:Paragraph 0847-0848

100-39-0
424 suppliers
$10.00/10g

140654-19-9
0 suppliers
inquiry