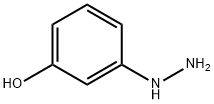
3-hydrazinylphenol synthesis
- Product Name:3-hydrazinylphenol
- CAS Number:793635-77-5
- Molecular formula:C6H8N2O
- Molecular Weight:124.14
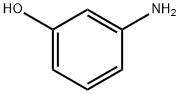
591-27-5
534 suppliers
$11.00/10g

793635-77-5
9 suppliers
inquiry
Yield:793635-77-5 16%
Reaction Conditions:
Stage #1: m-Hydroxyanilinewith hydrogenchloride;sodium nitrite in water at 10; for 0.166667 h;
Stage #2: with hydrogenchloride;tin(ll) chloride at 20; for 0.75 h;
Stage #3: with sodium hydroxide in water at 20; pH=10; for 0.75 h;
Steps:
20.a a. 3-Hydrazinophenol (Intermediate 20a)
To a stirred, ice cooled solution of 3-aminophenol (2.00 g, 18.3 mmol) in concentrated HC1 (20 mL) was added dropwise a solution of sodium nitrite (1.33 g, 19.24 mmol) in H20 (10 mL), ensuring the internal temperature did not exceed 10°C. After 10 min, a solution of tin (II) chloride (7.645 g, 40.3 mmol) in concentrated HC1 (10 mL) was added dropwise, and the ice bath removed. After stirring at RT for 45 min, the reaction was basified to pH 10 with careful addition of 10 M NaOH solution (50 mL), and then extracted with EtOAc (3 x). The organic extracts were dried over MgSC^, concentrated in vacuo and triturated with Et20 to afford the title compound as a brown solid (353 mg, 16%). 1H NMR (300 MHz, d6- DMSO): 3.83 (2H, br s), 5.96-6.02 (1H, m), 6.15-6.21 (1H, m), 6.22 (1H, t, J = 2.2 Hz), 6.52 (1H, br s), 6.84 (1H, t, J = 8.0 Hz), 8.91 (1H, br s).
References:
WO2014/195400,2014,A1 Location in patent:Page/Page column 134; 135

591-20-8
338 suppliers
$10.00/1g

793635-77-5
9 suppliers
inquiry