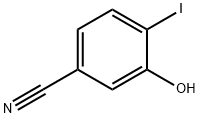
3-Hydroxy-4-iodobenzonitrile synthesis
- Product Name:3-Hydroxy-4-iodobenzonitrile
- CAS Number:210962-75-7
- Molecular formula:C7H4INO
- Molecular Weight:245.02
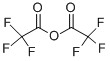
407-25-0
439 suppliers
$9.00/1g

210962-75-7
33 suppliers
inquiry
Yield:-
Reaction Conditions:
with pyridine;sodium hydroxide in tetrahydrofuran;1,4-dioxane;methanol;chloroform;ethyl acetate;
Steps:
R.1.2 Step 2
The residue was dissolved in 450 ml of dioxane. 17.4 ml (117 mmol) of trifluoroacetic acid anhydride and 21.8 ml (269 mmol) of pyridine were added to the obtained solution at 0° C. After stirring at room temperature for 18 hours, the solvent was evaporated under reduced pressure. The residue was treated with chloroform as an extracting solvent by an ordinary method to obtain an oily residue. The residue was dissolved in 180 ml of tetrahydrofuran/methanol (1/1). 90 ml (90.0 mmol) of 1 N aqueous sodium hydroxide solution was added to the obtained solution at room temperature. They were stirred for 4 hours, and the solvent was evaporated under reduced pressure. The residue was washed with dichloromethane. The product was acidified with 1 N hydrochloric acid and then treated with ethyl acetate as the extracting solvent by an ordinary method to obtain a crude product, which was purified by the silica gel column chromatography to obtain the title compound. Yield: 9.29 g (37.9 mmol) (42%) MS (FAB, m/z) 246 (MH+) H-NMR (CDCl3) δ 5.63 (1H, br), 6.96 (1H, dd), 7.23 (1H, d), 7.79 (1H, d)
References:
US2003/109547,2003,A1
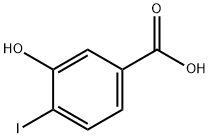
58123-77-6
194 suppliers
$6.00/250mg
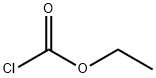
541-41-3
6 suppliers
$21.00/5g

210962-75-7
33 suppliers
inquiry

58123-77-6
194 suppliers
$6.00/250mg

210962-75-7
33 suppliers
inquiry
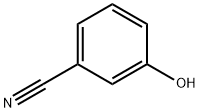
873-62-1
252 suppliers
$6.00/1g

210962-75-7
33 suppliers
inquiry
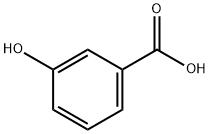
99-06-9
479 suppliers
$5.00/25g

210962-75-7
33 suppliers
inquiry