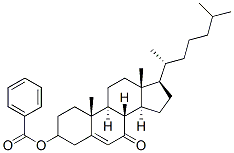
3-Hydroxy-cholest-5-en-7-one Benzoate synthesis
- Product Name:3-Hydroxy-cholest-5-en-7-one Benzoate
- CAS Number:6997-41-7
- Molecular formula:C34H48O3
- Molecular Weight:504.74

604-32-0
207 suppliers
$28.60/10MG

6997-41-7
12 suppliers
$272.60/1mg
Yield:6997-41-7 98%
Reaction Conditions:
with tert.-butylhydroperoxide;bis(acetylacetonato) oxovanadium(IV) in benzene at 20; for 120 h;Inert atmosphere;
Steps:
1 2.2. General procedure
General procedure: Substrate, 2.6 mmol (only 1.3 mmol for (5), (12), (13), and (14)for lack of solubility), and 0.38 mmol of VO(acac)2 were put into around bottom flask. The flask was then charged with 25 mL of benzene. After dissolving as much substrate as possible, 24 mmolof TBHP were added. The reaction flask was loosely capped to preventloss of solvent and the reaction was allowed to run for 5 days.At the end of 5 days, the benzene solvent was removed by evaporation.Residue was then extracted with deionized (DI) water andethyl ether into a separatory funnel. (If time is available, the precipitatewill dissolve into the solution overnight.) The ether layerwas retained and the ether was evaporated under reduced pressureat 55 C.Once the ether was removed, products (15) and (16) were dissolvedin acetone and recrystallized with DI water. The suspensionwas chilled for 5 h with an ice bath. Products (15) and (16) werethen filtered out, rinsed with DI water, and dried under reducedpressure with P2O5. The filtrate from the first recrystallization of(16) should be recrystallized again to extract more product.Beyond work-up of (15) and (16), we advise against recrystallizationfor the following products.After removing the ether, products (17-20) were separatedwith column chromatography (silica gel). Product (17) wasextracted with hexane into the column and eluted using a gradient(0-40% ethyl acetate in hexane). Also, product (18) was extractedwith 20% ethyl acetate in hexane and eluted with a gradient (20-50% ethyl acetate in hexane). Products (19) and (20) wereextracted into the column with 50:50 ethyl acetate and hexaneand were isocratically eluted with 50:50 ethyl acetate and hexane.Fractions were identified by TLC, collected, and dried underreduced pressure with P2O5. (We found that, particularly for elutionof (17), it is better get the products through the column as quickly as possible, without losing separation.)
References:
Grainger, Wendell S.;Parish, Edward J. [Steroids,2015,vol. 101,art. no. 7797,p. 103 - 109]
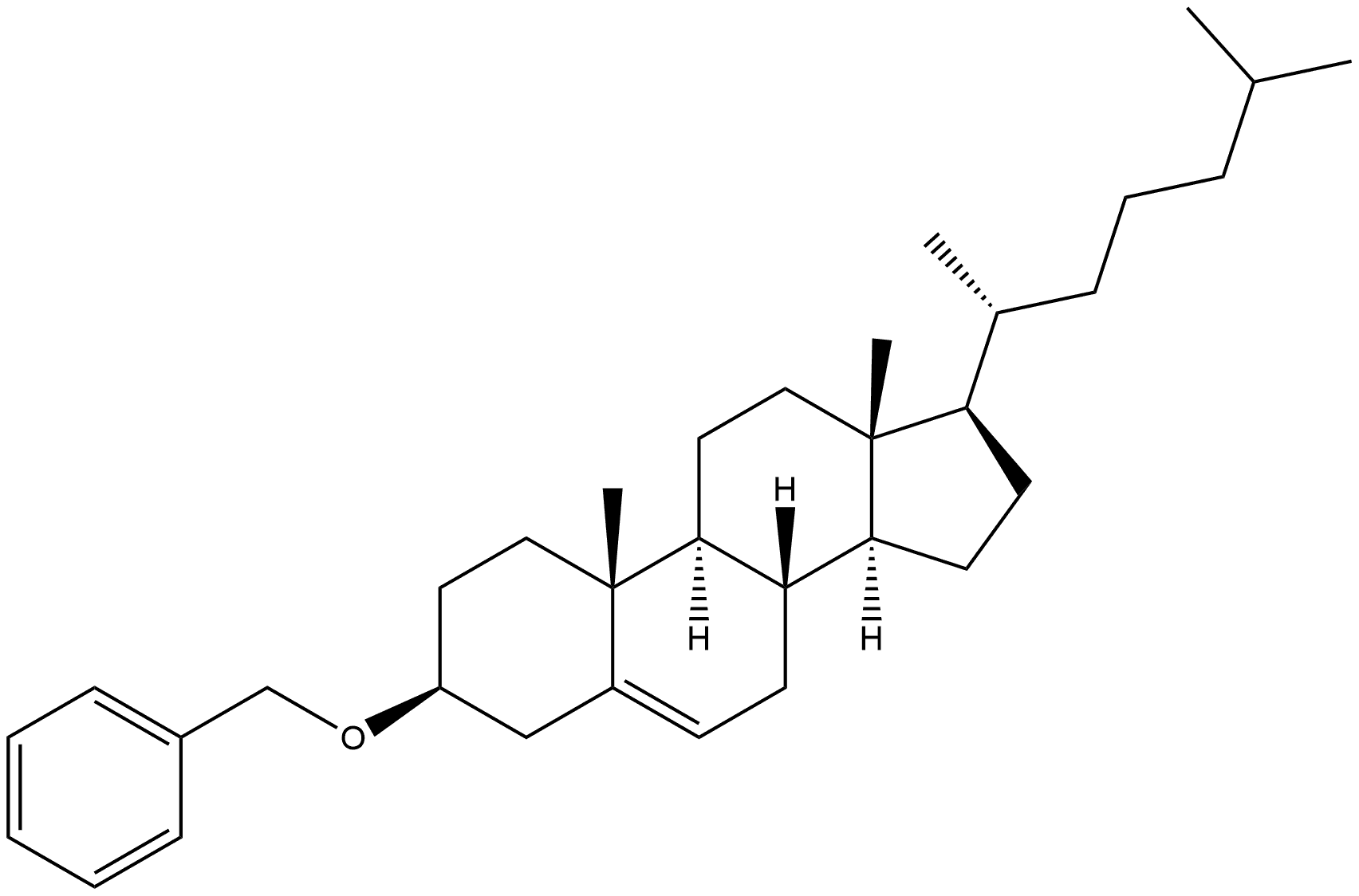
7278-60-6
0 suppliers
inquiry

6997-41-7
12 suppliers
$272.60/1mg
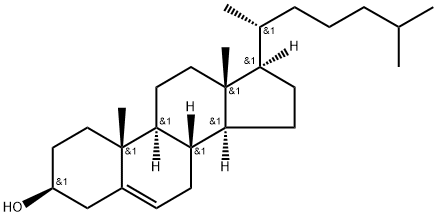
57-88-5
873 suppliers
$10.00/1g

6997-41-7
12 suppliers
$272.60/1mg
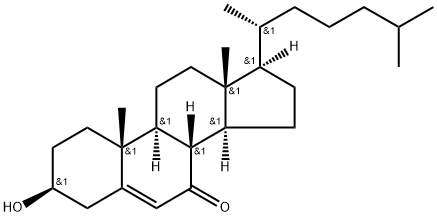
566-28-9
121 suppliers
$45.00/1mg
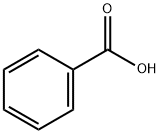
65-85-0
1444 suppliers
$10.00/25g

6997-41-7
12 suppliers
$272.60/1mg

566-28-9
121 suppliers
$45.00/1mg

98-88-4
597 suppliers
$10.00/5g

6997-41-7
12 suppliers
$272.60/1mg