
3-hydroxycyclopentanecarbonitrile synthesis
- Product Name:3-hydroxycyclopentanecarbonitrile
- CAS Number:194534-83-3
- Molecular formula:C6H9NO
- Molecular Weight:111.14

41171-91-9
41 suppliers
inquiry

194534-83-3
20 suppliers
inquiry
Yield:194534-83-3 95%
Reaction Conditions:
with sodium tetrahydroborate in methanol at 20; for 0.5 h;
Steps:
56.B Step B: 3-hydroxycyclopentanecarbonitrile
To a solution of 3-oxocyclopentanecarbonitrile (4 g, 37 mmol) in methanol (50 mL) at 0 ° C was added sodium borohydride(2 g, 44 mmol) and the reaction system was stabilized at 20-30 ° C. After 30 minutes of reaction at room temperature, the solvent was removed under reduced pressure and the residue was isolated by column chromatography (ethyl acetate / petroleum ether = 1/10) to give the title compound (3.8 g, 95%).
References:
CN107474024,2017,A Location in patent:Paragraph 0479; 0483; 0484; 0485
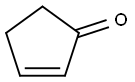
930-30-3
260 suppliers
$10.00/1g

194534-83-3
20 suppliers
inquiry

120-92-3
400 suppliers
$11.00/25g

194534-83-3
20 suppliers
inquiry