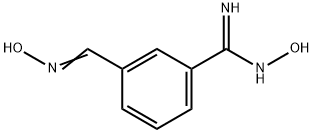
3-(HydroxyiMinoMethyl)benzaMidoxiMe, 97% synthesis
- Product Name:3-(HydroxyiMinoMethyl)benzaMidoxiMe, 97%
- CAS Number:1256486-34-6
- Molecular formula:C8H9N3O2
- Molecular Weight:179.18

24964-64-5
319 suppliers
$9.00/1g

1256486-34-6
9 suppliers
$28.00/1g
Yield:1256486-34-6 93.2%
Reaction Conditions:
Stage #1: 3-Cyanobenzaldehydewith hydroxylamine hydrochloride in ethanol;water at 20 - 25;Inert atmosphere;
Stage #2: with sodium carbonate in ethanol;water at 20 - 78;
Steps:
1a.1
A 5-L jacketed glass reactor equipped with an overhead mechanical stirrer, a thermocouple probe and a nitrogen purge was charged at 20-25 °C with 3-cyanobenzaldehyde (100.0 g,0.763 mol, 1.0 eq.) and ethanol (200 proof) (394.5 g, 500 mL, 5 v/w parts). To the suspension was charged via addition funnel, a solution of hydroxylamine hydrochloride (159.0 g, 2.288 mole, 3.0 eq.) in water (250 mL, 2.5 parts) over a period of 30-45 min while maintaining temperature of 20-25 °C. The addition funnel was rinsed with water (20 mL) and the rinse was added to the reactor. After addition of ca 45 mL of NH2OH.HCI solution, the solid dissolved to provide a clear solution. Within 10 min the solution turned cloudy and a solid crystallized to provide a suspension. The solid is believed to be the oxime resulting from addition of hydroxylamine to the aldehyde function. The suspension was stirred at 20-25 °C for 1 h. To the suspension was charged via a addition funnel, a solution of sodium carbonate (121.25 g, 1.144 mole, 1.5 eq,) in water (390 mL, 3.9 parts) over a period of 1.5-2.0 h while maintaining a temperature of 20-22 °C. The addition funnel was rinsed with water (20 mL) and the rinse was added to the reactor. Evolution of C02 was observed. The suspension was heated to 29- 30 °C and stirred at 29-30 °C for 24 h. Water (1.32 L, 13.2 parts) was charged to the reactor over 45-60 min while maintaining a temperature of 30-32 °C. The suspension was heated to and held at 76-78 °C for 30-60 min to get a clear solution. The solution was cooled to 55-60 °C over 90 min. Product crystallized at 55-60 °C. The suspension was stirred at 55-60 °C for 60 min. The suspension was cooled to 20-22 °C over 8-12 h. The suspension was cooled to 2- 5 °C and stirred at 2-5 °C for 4 h. The suspension was filtered (Buchner funnel, 14.5 cm o.d.) and the cake was washed with water (250 mL, 2.5 parts). The cake was dried under suction for 5 h. The cake was transferred to a drying dish and dried under vacuum (25-50 torr, 50 °C, N2) for 60 h to provide 127.33 g (93.2% yield) of product as a white crystalline solid with a purity of 99.9% (HPLC). HPLC Method: Zorbax Eclipse XDB C8 column, 5 micron, 4.6 x 150 mm, 25 °C, detection at 240 nm, gradient: 5:95:0.1 CH3CN/H20/TFA isocratic 2 min then ramped over 16 min to 90:10:0.1 CH3CN/H20/TFA; product retention time: 3.6-4.4 min (three peaks)
References:
WO2011/44307,2011,A1 Location in patent:Page/Page column 25-26