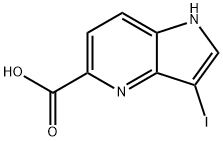
3-Iodo-4-azaindole-5-carboxylic acid synthesis
- Product Name:3-Iodo-4-azaindole-5-carboxylic acid
- CAS Number:1190311-30-8
- Molecular formula:C8H5IN2O2
- Molecular Weight:288.04
![1H-PYRROLO[3,2-B]PYRIDINE-5-CARBOXYLIC ACID](/CAS/GIF/872355-64-1.gif)
872355-64-1
69 suppliers
$45.00/10mg

1190311-30-8
9 suppliers
inquiry
Yield:1190311-30-8 72.6%
Reaction Conditions:
with iodine;potassium hydroxide in N,N-dimethyl-formamide at 20; for 1 h;
Steps:
9
Preparation of compound 9a: 3-iodo-lH-pyrrolo[3,2-b]pyridine-5-carboxylic acidTo a solution of lH-pyrrolo[3,2-b]pyridine-5-carboxylic acid (0.400 g, 2.467 mmol, Adesis, New Castle, DE) in DMF (4.9 mL) was added I2 (0.626 g, 2.467 mmol) and KOH (0.346 g, 6.17 mmol). The mixture was stirred at RT for 1 h. The mixture was poured into ice and H20 (30 mL) containing sodium bisulfite (0.257 g, 2.467 mmol). The reaction was acidified with 5 M HCl. A yellow solid precipitated out and was collected by filtration, washed with H20 and dried overnight in a vacuum oven to give 3-iodo-lH- pyrrolo[3,2-b]pyridine-5-carboxylic acid (0.516 g, 1.791 mmol, 72.6 % ). MS (ESI, pos. ion) m/z: 289.0 (M+l). .H NMR (400 MHz, DMSO-d6) δ ppm 7.91 (2 H, s), 8.01 (1 H, d, J=2.74 Hz), 12.03 - 12.11 (1 H, m).
References:
WO2012/129338,2012,A1 Location in patent:Page/Page column 84