3-IODO-6,7-DIHYDRO-5H-PYRAZOLO[5,1-B][1,3]OXAZINE synthesis
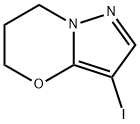
- Product Name:3-IODO-6,7-DIHYDRO-5H-PYRAZOLO[5,1-B][1,3]OXAZINE
- CAS Number:1383675-85-1
- Molecular formula:C6H7IN2O
- Molecular Weight:250.04
![6,7-DIHYDRO-5H-PYRAZOLO[5,1-B][1,3]OXAZINE](/CAS/20180527/GIF/1383675-84-0.gif)
1383675-84-0
22 suppliers
$220.00/100mg
![3-IODO-6,7-DIHYDRO-5H-PYRAZOLO[5,1-B][1,3]OXAZINE](/CAS/20180629/GIF/CB24001324.gif)
1383675-85-1
18 suppliers
inquiry
Yield:1383675-85-1 62.2%
Reaction Conditions:
with N-iodo-succinimide in acetonitrile at 17 - 25; for 1 h;
Steps:
8 Preparation of 3-iodo-6,7-dihydro-5H-pyrazolo[5,1-b][1,3]oxazine
To a solution of 6,7-dihydro-5H-pyrazolo[5,1-b][1,3]oxazine (83.0 g, 669.3 mmol) in CH3CN (1500 mL) was added N-iodosuccinimide (155.0 g, 688.8 mmol). The mixture was stirred at r.t. for 1 h before being slowly poured into vigorously stirred water (1000 mL). Saturated aqueous sodium thiosulphate (500 mL) was added, and the resulting mixture was extracted with ethyl acetate (2×800 mL). The combined organic layers were washed with water, dried over Na2SO4, and concentrated under reduced pressure. The resulting residue was purified by column chromatography on silica gel (petroleum ether: EtOAc=1:1) to give 3-iodo-6,7-dihydro-5H-pyrazolo[5,1-b][1,3]oxazine (105.0 g, 62.2%) as a yellow solid. 1H NMR (300 MHz, DMSO-d6, 30° C.) 2.19-2.12 (2H, m), 4.10-3.98 (2H, m), 4.34-4.31 (2H, m), 7.28 (1H, m). m/z: ES+[M+H]+ 251.
References:
US2016/376287,2016,A1 Location in patent:Paragraph 0638