
3-IODOBENZYL ALCOHOL synthesis
- Product Name:3-IODOBENZYL ALCOHOL
- CAS Number:57455-06-8
- Molecular formula:C7H7IO
- Molecular Weight:234.03
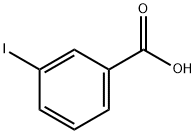
618-51-9
380 suppliers
$9.00/10g

57455-06-8
152 suppliers
$19.00/1g
Yield:57455-06-8 54%
Reaction Conditions:
Stage #1:3-Iodobenzoic acid with chloroformic acid ethyl ester;triethylamine in tetrahydrofuran at -8; for 0.5 h;
Stage #2: with sodium tetrahydroborate in tetrahydrofuran;water at 10 - 20; for 22 h;
Stage #3: with hydrogenchloride in tetrahydrofuran;water; pH=1
Steps:
1
3-Iodobenzvl alcohol (11) Ethyl chloroformate (1.02 g, 11.1 mmol) in dry tetrahydrofuran (5 ml) was added slowly over 10 min to a stirred solution of 3-iodobenzoic acid (1.98 g, 7.98 mmol) and triethylamine (0.500 g, 11.6 mmol) in dry tetrahydrofuran at-8° (ice/salt/water bath). The yellow mixture soon became cloudy. It was shielded from light and stirred at-8° for 0.5 h, after which it was filtered to remove the triethylammonium chloride precipitate. The filtrate was added slowly to a suspension of sodium borohydride (0.795 g, 21.1 mmol) in water (30 ml) in a three necked flask, equipped with a thermometer, and connected to nitrogen, making sure that the temperature of the mixture is kept below 10° during the addition. Gas evolution (carbon dioxide) was observed. After the addition was completed, the mixture was stirred, shielded from light, at room temperature for 22 h under nitrogen. The resultant cloudy mixture was acidified with aqueous hydrochloric acid (10%) to pH- 1. The layers were separated, and the aqueous layer was extracted with ether (x 3). The combined ether extract and tetrahydrofuran layer was washed with aqueous sodium hydroxide (x 1, 10%) and then with water (x 1). Drying with magnesium sulphate, and concentration gave 3-iodobenzyl alcohol (11) a pale yellow oil (1.02 g, 54%). IH nmr (400 MHz, CDC13) : 64. 66 (s, 2H) CH2 ; 7.10 (brt, J 7. 7 Hz, 1H) H5; 7.32 (brd, J 7.7 Hz, 1H) H6,7. 63 (brd, J 7. 7 Hz, 1H) H4; 7.74 (brs, 1H) H2.
References:
PETER MACCALLUM CANCER INSTITUTE WO2005/82894, 2005, A1 Location in patent:Page/Page column 41
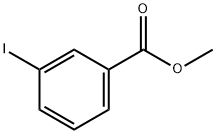
618-91-7
138 suppliers
$11.00/5g

57455-06-8
152 suppliers
$19.00/1g
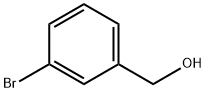
15852-73-0
274 suppliers
$11.00/25g

57455-06-8
152 suppliers
$19.00/1g
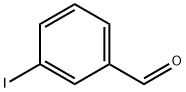
696-41-3
231 suppliers
$7.00/250mg

57455-06-8
152 suppliers
$19.00/1g

618-51-9
380 suppliers
$9.00/10g