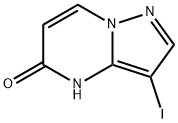
3-Iodopyrazolo[1,5-a]pyrimidin-5(4H)-one synthesis
- Product Name:3-Iodopyrazolo[1,5-a]pyrimidin-5(4H)-one
- CAS Number:1224944-44-8
- Molecular formula:C6H4IN3O
- Molecular Weight:261.02
![5-Hydroxypyrazolo[1,5-a]pyrimidine](/CAS/GIF/29274-22-4.gif)
29274-22-4
142 suppliers
$9.00/100mg
![3-Iodopyrazolo[1,5-a]pyrimidin-5(4H)-one](/CAS/20210111/GIF/1224944-44-8.gif)
1224944-44-8
3 suppliers
inquiry
Yield:1224944-44-8 86%
Reaction Conditions:
with N-iodo-succinimide in N,N-dimethyl-formamide at 20;Inert atmosphere;
Steps:
1
Pyrazolo[l,5-α]pyrimidin-5(4H)-one (1.0 g, 7.4 mM, 1.0 equiv) and N- iodosuccinimide (1.67 g, 7.4 mM, 1.0 equiv) were combined with 20 mL of DMF and warmed slightly. Within minutes a heavy precipitate formed. The reaction mixture was stirred an additional 1 h at ambient temperature then cooled on an icewater bath and filtered. The collected solid was washed with 20 mL of dichloromethane and air dried to give 1.66 g (86%) of 3-iodopyrazolo[l,5-α]pyrimidin-5(4H)-one. LCMS (ESI) m+H = 262.2; 1H NMR (400 MHz, OMSO-Ct6) δ: 12.1 (s, 1 H), 8.5 (d, 1 H, J=7.6), 7.84 (s, 1 H), 6.01 (d, 1 H, J=7.1).
References:
WO2010/51549,2010,A1 Location in patent:Page/Page column 42; 75-76
![4H-Pyrazolo[1,5-a]pyrimidin-5-one](/CAS/20200331/GIF/CB62491587.gif)
1224944-43-7
8 suppliers
inquiry
![3-Iodopyrazolo[1,5-a]pyrimidin-5(4H)-one](/CAS/20210111/GIF/1224944-44-8.gif)
1224944-44-8
3 suppliers
inquiry