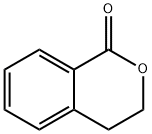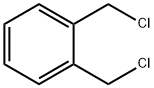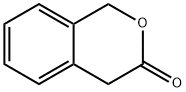
3-Isochromanone synthesis
- Product Name:3-Isochromanone
- CAS Number:4385-35-7
- Molecular formula:C9H8O2
- Molecular Weight:148.16

91-13-4
240 suppliers
$14.00/1g

4385-35-7
284 suppliers
$6.00/5g
Yield:4385-35-7 95.1%
Reaction Conditions:
in tert-butyl alcohol
Steps:
8 EXAMPLE 8
EXAMPLE 8 The procedure of Example 6 was repeated, except for replacing α,α'-o-xylene dichloride with 13.2 g (0.05 mol) of α,α'-o-xylene dibromide and replacing butyl alcohol with 20 g of t-butyl alcohol. As a result, 2.9 g (yield: 39.2%) of 3-isochromanone was obtained. The purity of the product was 95.1% as measured by gas chromatographic analysis.
References:
Sagami Chemical Research Center;Iharanikkei Chemical Industry Co., Ltd US5886211, 1999, A

95335-46-9
68 suppliers
$27.00/250mg

4385-35-7
284 suppliers
$6.00/5g
201230-82-2
1 suppliers
inquiry
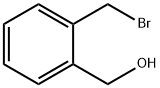
74785-02-7
42 suppliers
$35.00/100mg

4385-35-7
284 suppliers
$6.00/5g
![2-[2-(hydroxyMethyl)phenyl]ethanol](/CAS/20150408/GIF/6346-00-5.gif)