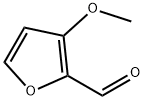
3-Methoxyfuran-2-carbaldehyde synthesis
- Product Name:3-Methoxyfuran-2-carbaldehyde
- CAS Number:32487-58-4
- Molecular formula:C6H6O3
- Molecular Weight:126.11
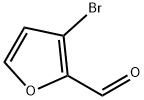
14757-78-9
105 suppliers
$21.00/250mg
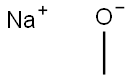
124-41-4
680 suppliers
$12.00/25g

32487-58-4
87 suppliers
$49.00/100mg
Yield: 59%
Reaction Conditions:
in methanol at 20 - 64; for 18 h;Inert atmosphere;Reflux;
References:
Ronn, Magnus;Lim, Ngiap-Kie;Hogan, Philip;Zhang, Wu-Yan;Zhu, Zhijian;Dunwoody, Nicholas [Synlett,2012,# 1,art. no. S51311ST,p. 134 - 136] Location in patent:experimental part