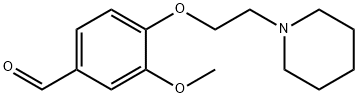
3-METHOXY-4-(2-PIPERIDIN-1-YL-ETHOXY)-BENZALDEHYDE synthesis
- Product Name:3-METHOXY-4-(2-PIPERIDIN-1-YL-ETHOXY)-BENZALDEHYDE
- CAS Number:46995-88-4
- Molecular formula:C15H21NO3
- Molecular Weight:263.33

110-89-4
3 suppliers
$15.96/100ML

204915-71-9
17 suppliers
inquiry

46995-88-4
13 suppliers
$353.00/1g
Yield:46995-88-4 91.07 %
Reaction Conditions:
with tetrabutylammomium bromide;potassium carbonate;potassium iodide in acetonitrile;
Steps:
4.1.5. General method of obtaining intermediates 7a-7d (method B)
General procedure: 3-Hydroxy-4-methoxybenzaldehyde (1 eq), K2CO3 (1 eq), KI (0.1 eq),the corresponding small molecule amine (2 eq) were dissolved in anhydrous acetonitrile. The mixture was stirred for 11 h at refllux. The reaction mixture was quenched with water (30.00 mL) and extracted with EtOAc (3 × 40.00 mL). The organic phases were combined, dried over Na2SO4, filtered and concentrated under reduced pressure to provide product.
References:
Xu, Huashen;Wang, Jianmin;Chen, Yuanguang;Du, Yang;Chen, Lu;Wu, Chunfu;Wang, Lihui;Chen, Guoliang [European Journal of Medicinal Chemistry,2023,vol. 250,art. no. 115171]

110-89-4
3 suppliers
$15.96/100ML
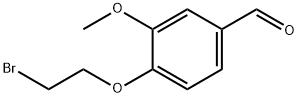
99070-23-2
18 suppliers
$34.00/1g

46995-88-4
13 suppliers
$353.00/1g

2008-75-5
264 suppliers
$10.00/1g

121-33-5
1092 suppliers
$9.00/1g

46995-88-4
13 suppliers
$353.00/1g

121-33-5
1092 suppliers
$9.00/1g

46995-88-4
13 suppliers
$353.00/1g
