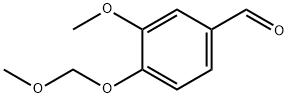
3-methoxy-4-(methoxymethoxy)benzaldehyde synthesis
- Product Name:3-methoxy-4-(methoxymethoxy)benzaldehyde
- CAS Number:5533-00-6
- Molecular formula:C10H12O4
- Molecular Weight:196.1999
Yield:5533-00-6 98%
Reaction Conditions:
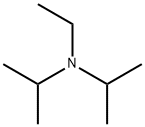
with N-ethyl-N,N-diisopropylamine in dichloromethane; for 0.75 h;Inert atmosphere;Cooling with ice;
Steps:
4.14 Preparation of 3-methoxy-4-methoxymethoxybenzaldehyde 7e
An ice cooled stirred solution of 3-methoxy-4-hydroxybenzaldehyde (9.13 g, 60 mmol) and N,N-diisopropylethylamine (21 mL, 120 mmol) in anhydrous methylene chloride (400 mL) under nitrogen was treated dropwise with bromomethyl methyl ether (6.0 mL, 66 mmol corrected for 90%) and stirred on an ice bath for 45 min. Aqueous sodium hydroxide (1 M, 150 mL) was added and the mixture stirred for a few minutes and separated. The aqueous solution was extracted with dichloromethane (100 mL) and the combined organic solution washed with water (200 mL), dried (Na2SO4), and concentrated in vacuo. The residue was dissolved in minimum dichloromethane and loaded onto a silica gel column and eluted with 1.5% ethyl acetate/dichloromethane to afford 3-methoxy-4-methoxymethoxybenzaldehyde (11.56 g, 98%) as a colorless oil: LC/MS m/z 197 [M+H]; 1H NMR (CDCl3, 400 MHz) δ 3.52 (s, 3H), 3.95 (s, 3H), 5.32 (s, 2H), 7.27 (d, J = 8.2, 1H), 7.41-7.45 (m, 2H), 9.87 (s, 1H); 13C NMR (CDCl3, 100 MHz) δ 56.2, 56.6, 95.2, 109.8, 115.0, 126.4, 131.3, 150.3, 152.2, 191.0. Anal. Calcd for C10H12O4: C, 61.22; H, 6.16. Found: C, 60.94; H, 6.23.
References:
Zhang, Mingbao;Erik Jagdmann Jr.;Van Zandt, Michael;Beckett, Paul;Schroeter, Hagen [Tetrahedron Asymmetry,2013,vol. 24,# 7,p. 362 - 373]

121-33-5
1187 suppliers
$9.00/1g
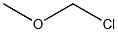
107-30-2
0 suppliers
$110.00/2.5g
7087-68-5
606 suppliers
$9.00/1g

5533-00-6
6 suppliers
inquiry
139-85-5
847 suppliers
$5.00/1g

5533-00-6
6 suppliers
inquiry

![Benzaldehyde, 4-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-3-methoxy-](/CAS/20180601/GIF/69404-94-0.gif)