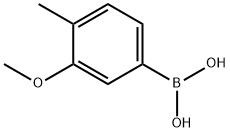
3-Methoxy-4-methylbenzeneboronic acid, 97% synthesis
- Product Name:3-Methoxy-4-methylbenzeneboronic acid, 97%
- CAS Number:917757-15-4
- Molecular formula:C8H11BO3
- Molecular Weight:165.98

5419-55-6
379 suppliers
$12.00/5g
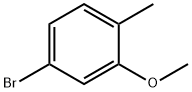
67868-73-9
89 suppliers
$34.60/1g

917757-15-4
108 suppliers
$30.00/1 G
Yield: 97%
Reaction Conditions:
Stage #1:5-bromo-2-methylphenol methyl ether with n-butyllithium in tetrahydrofuran;hexane at -78; for 0.75 h;
Stage #2:Triisopropyl borate in tetrahydrofuran;hexane at -78 - -30;
Stage #3: with hydrogenchloride in tetrahydrofuran;hexane;water at -30 - 20;
Steps:
General procedure: nButyllithium (1.6 M in hexanes, 3.75 mL) was added dropwise to a stirred solution ofbromophenol ether (5 mmol) in tetrahydrofuran (20 mL) at -78 °C for 45 min before theaddition of triisopropyl borate (1.75 mL, 7.5 mmol). The reaction was allowed to slowlywarm to -30 °C before the addition of 2.5 N hydrochloric acid (10 mL). The mixture waswarmed to ambient temperature while stirring and diluted with ethyl acetate. The layerswere separated and the aqueous layer was extracted twice with the latter solvent. Thecombined organic layers were washed with brine, dried over sodium sulfate, filtered,and concentrated in vacuo. The crude material was purified using flash columnchromatography (gradient 0-40% ethyl acetate/hexanes) to give the title compounds aswhite powders.
References:
Binder, Randall J.;Hatfield, M. Jason;Chi, Liying;Potter, Philip M. [European Journal of Medicinal Chemistry,2018,vol. 149,p. 79 - 89] Location in patent:supporting information