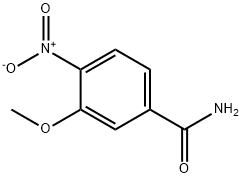
3-Methoxy-4-nitrobenzamide synthesis
- Product Name:3-Methoxy-4-nitrobenzamide
- CAS Number:92241-87-7
- Molecular formula:C8H8N2O4
- Molecular Weight:196.16
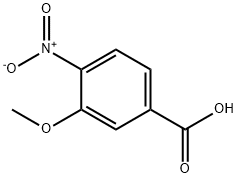
5081-36-7
223 suppliers
$6.00/1g

92241-87-7
31 suppliers
inquiry
Yield:92241-87-7 97%
Reaction Conditions:
Stage #1: 3-methoxy-4-nitrobenzoic acidwith oxalyl dichloride;N,N-dimethyl-formamide in tetrahydrofuran at 0 - 20; for 1 h;
Stage #2: with ammonia in tetrahydrofuran at 0; for 0.166667 h;
Steps:
1.a
3-(Methyloxy)-4-nitrobenzonitrile. Following the procedure of Mackman et al. in J. Med. Chem. 2001, 44, 2753-2771 , 3-methoxy-4-nitrobenzoic acid (11.52 g, 58.4 mmol) was dissolved in THF (158 mL) and cooled to 0 DC. Oxalyl chloride (5.6 mL, 64.3 mmol) was added dropwise under a nitrogen atmosphere, followed by a few drops of DMF. The reaction mixture was allowed to warm to room temperature and stirred for 1 h. The mixture was then concentrated to dryness under reduced pressure, and the residue redissolved in THF (158 mL) and cooled to 0 DC. Ammonia gas was bubbled through the solution for 10 min, leading to the formation of a yellow precipitate. The ice bath was removed, and the mixture was sealed and allowed to stir overnight. After the addition of EtOAc (100 mL), the solids were filtered off, washed with water, and dried to provide 3-(methyloxy)-4-nitrobenzamide (10.10 g, 88%) as a yellow solid. Additional product could be recovered from the filtrate by removal of the organic solvent under reduced pressure, then redissolving the residue in EtOAc. The organic layer was washed with 1 N HCI (2 x 100 mL), brine (2 x 100 mL), then dried (Na2SO4), filtered and concentrated to afford an additional 1.07 g (9%). 1 H NMR (d6-DMSO): D 8.25 (bs, 1 H), 7.96 (d, J = 8.0 Hz, 1 H), 7.75 (d, J = 1.6 Hz, 1 H), 7.73 (s, 1 H), 7.57 (dd, J = 1.6, 8.4 Hz, 1 H), 3.98 (s, 3H).
References:
WO2007/103755,2007,A2 Location in patent:Page/Page column 48; 56
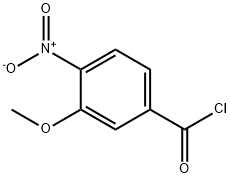
67579-92-4
8 suppliers
inquiry

92241-87-7
31 suppliers
inquiry
5081-37-8
124 suppliers
$50.10/1g

92241-87-7
31 suppliers
inquiry
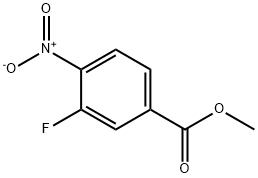
185629-31-6
171 suppliers
$5.00/250mg

92241-87-7
31 suppliers
inquiry
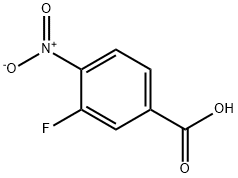
403-21-4
258 suppliers
$6.00/1g

92241-87-7
31 suppliers
inquiry