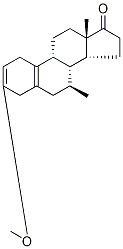
3-Methoxy-7α-Methyl-estra-2,5(10)-dien-17-one synthesis
- Product Name:3-Methoxy-7α-Methyl-estra-2,5(10)-dien-17-one
- CAS Number:5210-25-3
- Molecular formula:C20H28O2
- Molecular Weight:300.44
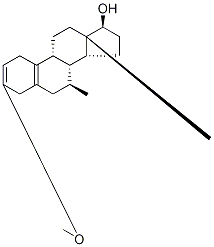
15506-02-2
7 suppliers
$2440.00/200mg

5210-25-3
7 suppliers
$120.00/10mg
Yield:5210-25-3 84.5%
Reaction Conditions:
with aluminum tri-tert-butoxide;benzaldehyde;2,6-di-tert-butyl-4-methyl-phenol in tert-butyl methyl ether at 20; for 1 h;
Steps:
6 Example 6
TERT-BUTYL METHYL ether (222.0 g), 2, 6-DI-TERT-BUTYL-4-METHYLPHENOL (0.50 g), compound (8A) (50 g) and BENZALDEHYDE (26.3 g) were combined at ambient temperature and vacuum degassed with nitrogen. Aluminium tri-tert-butoxide (8. 14 g) was then added and the hazy solution stirred for 60 minutes. The reaction was sampled after this period and then at 30 minute intervals until complete by HPLC analysis. Once complete, an aqueous solution of lactic acid (16. 92 G in 250 g water) was added and the resulting biphasic solution stirred for at least 15 minutes. The organic layer was separated, washed successively with a 5% w/w solution of sodium chloride (263. 2 G), 5% w/v solution of sodium hydrogen carbonate (105. 0 g) and finally with water. The solution was concentrated by distillation until 2 volumes of methyl test-butyl ether had been collected with respect TO THE INPUT WEIGHT OF COMPOUND (8A). Methanol (197. 8 G) WAS THEN ADDED AND DISTILLATION CONTINUED UNTIL A FURTHER 5 volumes [with respect to the input weight of compound (8A)] of distillate had been collected. The solution was then cooled to 46-54 C and crystallisation initiated. The suspension was then chilled at 0 to-5°C for a further 60 minutes. The product was isolated by filtration, washed firstly with methanol, and secondly with aqueous methanol. The product was dried in the vacuum oven to constant weight (yield : 84. 5%).
References:
WO2004/78774,2004,A1 Location in patent:Page 37

10449-00-0
0 suppliers
inquiry

5210-25-3
7 suppliers
$120.00/10mg
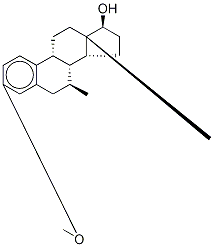
15506-01-1
5 suppliers
$650.00/20mg

5210-25-3
7 suppliers
$120.00/10mg