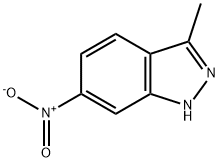
3-Methyl-6-nitroindazole synthesis
- Product Name:3-Methyl-6-nitroindazole
- CAS Number:6494-19-5
- Molecular formula:C8H7N3O2
- Molecular Weight:177.16
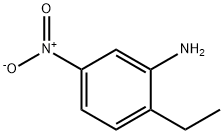
20191-74-6
134 suppliers
$6.00/250mg

6494-19-5
259 suppliers
$19.00/1g
Yield:6494-19-5 98%
Reaction Conditions:
with tert.-butylnitrite in glacial acetic acid at 20; for 0.75 h;Inert atmosphere;
Steps:
1 Intermediate Example 1
Preparation of 3-methyl-1H-indazol-6-amine
Intermediate Example 1
Preparation of 3-methyl-1H-indazol-6-amine
To a solution of 10 g (.06 mol) of 2-ethyl-5-nitroaniline (prepared by nitration of 2-ethylaniline:) in 300 ml of glacial acetic acid, at room temperature, was added a solution of 8.98 ml (.06 mol) of tert-butyl nitrite in 40 ml of acetic acid dropwise over 15 min.
After the addition was complete the solution was allowed to stir for 30 min.
The acetic acid was removed in vacuo to afford an orange solid.
The solid was dissolved in approximately 120 ml of ethyl acetate and washed with 3 x 100 ml sat. aqueous NaHCO3.
The organic layer was dried over MgSO4 and the solvent was removed in vacuo to afford 3-methyl-6-nitroindazole as a yellow solid (10.4 g, 98%).
To a stirred solution of 10 g (.06 mol) of 3-methyl-6-nitroindazole in 100 ml of 2-methoxyethyl ether, at 0 °C, was added a solution of 45 g (.24 mol) of tin(II) chloride in 86 ml of concentrated HCl dropwise over 15 min, in order to keep the reaction temperature below 100 °C.
After the addition was complete, the ice bath was removed and the solution was allowed to stir for an additional 20 min.
Approximately 70 ml of diethyl ether was added to reaction, resulting in precipitate formation. The resulting precipitate was isolated by filtration and washed with diethyl ether, and afforded a yellow solid (10 g, 92 %), the HCl salt of 3-methyl-1H-indazol-6-amine.
References:
EP2311825,2015,B1 Location in patent:Paragraph 0157 - 0159
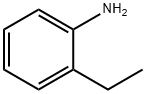
578-54-1
257 suppliers
$10.00/5g

6494-19-5
259 suppliers
$19.00/1g