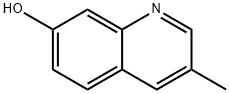
3-methyl-7-Quinolinol synthesis
- Product Name:3-methyl-7-Quinolinol
- CAS Number:851985-87-0
- Molecular formula:C10H9NO
- Molecular Weight:159.18
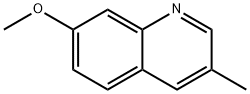
851985-86-9
4 suppliers
inquiry

851985-87-0
14 suppliers
$356.69/250mg
Yield:851985-87-0 84%
Reaction Conditions:
with water;hydrogen bromide for 120 h;Heating / reflux;
Steps:
27
Description 27; 3-Methvlquinolin-7-ol; A mixture of Description 26 (6.0 g, 34.6 mmol) and 48% aqueous HBr (150 ml) was heated at reflux for 5 days. The mixture was cooled and basified by the careful addition of 33% aqueous ammonia. The resulting precipitate was removed by filtration, washed with water, and dried in-vacuo to give the title compound as a pink solid (4.6 g, 84%). 1H NMR (400 MHz, DMSO-d6) 2. 41 (3 H, s), 7.13 (1 H, dd, J8. 8 and 2.4), 7.22 (1 H, d, J2.4), 7.71 (1 H, d, J8. 8), 7.96 (1 H, t, J0. 7), 8.61 (1 H, d, J2.2), 10. 01 (1 H, s).
References:
WO2005/47279,2005,A1 Location in patent:Page/Page column 35