
3-METHYLBUT-3-ENYLBENZOATE synthesis
- Product Name:3-METHYLBUT-3-ENYLBENZOATE
- CAS Number:5205-12-9
- Molecular formula:C12H14O2
- Molecular Weight:190.24
Yield:5205-12-9 100%
Reaction Conditions:
with triethylamine in dichloromethane at 0; for 0.666667 h;
Steps:
55
Example 55 3-methylbut-3-enyl benzoate (47) In a 250 ml flask, in a nitrogen atmosphere, 3-methylbut-3-enole (10 g, 116 mmol, 1 eq) dissolved in 100 ml of dichloromethane is introduced, followed by triethylamine (23.5 g, 236 mmol, 2 eq) at -0° C. Benzoyl chloride (24.4 g, 174 mmol, 1.5 eq) in solution in 20 ml of dichloromethane is then added over a 10 min period. After 30 min of reaction, the temperature is increased to 25° C. and the organic phase washed with iced water (100 ml), 10% hydrochloric acid (100 ml) and with a saturated NaHCO3 solution. After drying on sodium sulphates and evaporation of the dichloromethane, the residual oil obtained undergoes silica gel chromatography (4:6 ethyl ether:petroleum ether) to produce a colourless oil. Total formula: C12H14O2 Yield=100% Rf=0.70 (4:6, ethyl ether:petroleum ether). NMR 1H(CDCL3), δ(ppm): 1.84 (S, 3H, H-5), 2.51 (t, J2-1 6.7 Hz, 2H, H-2), 4.47 (t, J1-2 6.7 Hz, 2H, H-1), 4.82-4.92 (m, 2H, H-4), 7.40-7.50 (m, 2H, H-ar), 7.52-7.65 (m, 1H, H-ar), 8.02-8.12 (m, 2H, H-ar). NMR 13C(CDCL3), δ(ppm): 22.96 (1C, C-5), 37.24 (1C, C-2), 63.59 (1C, C-1), 112.85 (1C, C-4), 128.76-129.80 (4C, C-ar), 130.82 (1C, C-C=O), 133.28 (1C, C-ar), 142.15 (1C, C-3), 166.99 (1C, C-carbonyl). MS FAB>0 m/z (NOBA): 191 [M+H]+; 212 [M+Na]+.
References:
US2006/241087,2006,A1 Location in patent:Page/Page column 22-23
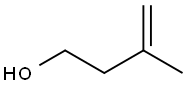
763-32-6
214 suppliers
$9.00/25g

98-88-4
586 suppliers
$10.00/5g
624-96-4
22 suppliers
inquiry
10523-96-3
13 suppliers
inquiry
60044-31-7
0 suppliers
inquiry

5205-12-9
4 suppliers
inquiry
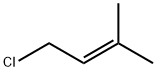
503-60-6
172 suppliers
$22.00/1g