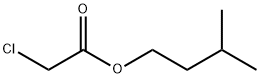
3-methylbutyl chloroacetate synthesis
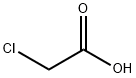
- Product Name:3-methylbutyl chloroacetate
- CAS Number:5326-92-1
- Molecular formula:C7H13ClO2
- Molecular Weight:164.63
Yield:5326-92-1 78%
Reaction Conditions:
with sulfuric acid for 6 h;Reflux;Fischer–Speier Esterification;
Steps:
General procedure for obtaining alkyl 2-chloroacetates via Fisher esterification (5a-5h)
General procedure: A mixture of 2-chloroacetic acid (20 mmol), the respective alcohol (4a-4h) (60 mL) and concentrated sulfuric acid (1 mL) was refluxed for 6 h. Afterwards,the excess solvent was rotary-evaporated, and the residue poured into cold water. The residue was transferred to a separation funnel containing 250 mL of water, and 100 mLof ethyl ether were then added. The organic phase was separated, washed repeatedly with 10% sodium bicarbonate until neutral pH and then dried with anhydrous NaSO4. Ethyl ether was rotary-evaporated, obtaining the respective esters (5a-5h).
References:
Barbosa-Filho, José M.;Brand?o, Maria Cláudia R.;Lima, Edeltrudes O.;Lira, Bruno F.;Neto, Hermes D.;Souza, Helivaldo D. S.;Trindade, Emmely O.;de Athayde-Filho, Petr?nio F. [Journal of the Brazilian Chemical Society,2020,vol. 31,# 8,p. 1668 - 1678]
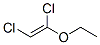
42345-82-4
1 suppliers
inquiry

123-51-3
519 suppliers
$25.00/25mL

5326-92-1
12 suppliers
inquiry
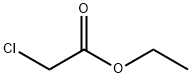
105-39-5
380 suppliers
$10.00/5g