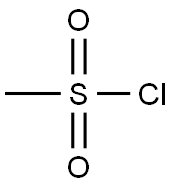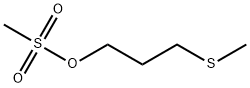
3-(Methylthio)propyl (Methanesulfonate) synthesis
- Product Name:3-(Methylthio)propyl (Methanesulfonate)
- CAS Number:232944-38-6
- Molecular formula:C5H12O3S2
- Molecular Weight:184.28
Yield:232944-38-6 79%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 0.5 h;
Steps:
2.1 3-(methylthio)methanesulfonic acid propyl ester
3-(methylthio)propan-1-ol(2mL, 19.4mmol), triethylamine(3.2 mL, 23 mmol) was dissolved in dichloromethane (30 mL), and methanesulfonyl chloride (1.84 g) was added dropwise under an ice bath, and the mixture was stirred at room temperature for 1 hour. ML, 23.3 mmol).After completion of the reaction, the reaction solution was stirred at room temperature for 30 minutes.The reaction solution was quenched with saturated aqueous sodium bicarbonate solution (30 mL) and extracted with dichloromethane (50 mL x 2). The organic phases were combined, washed with saturated sodium chloride solution (50 mL), dried over anhydrous sodium sulfate, The filtrate was concentrated under reduced pressure.The residue was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 1: 1) to give the title compound (7.8 g, yield 79%) as a pale yellow oil.
References:
CN104262330,2016,B Location in patent:Paragraph 0186-0190